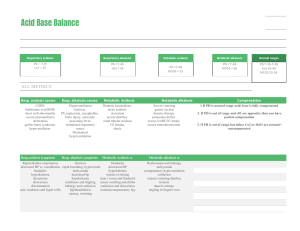
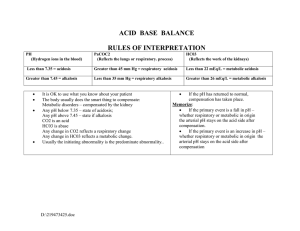
Acid Base Imbalances + ABGs Pathophysiology Course Pathophysiology pH Acid base imbalances are the balance of Acid & Base in the body, kind of like a tug of war the body loves to keep pH in balance. - NORMAL pH NORMAL pH Acidotic 7.35 pH 7.35 pH Alkalotic 7.45 pHpH 7.45 Normal pH: 7.35 - 7.45 Acidosis: Less than 7.35 pH Alkalosis (base): Over 7.45 pH • Full compensation = FULLY Normal pH 7.35 - 7.45 • Partial compensation = pH is not normal MEMORY TRICKS Base = Bicarbonate Controlling Organs Lungs control Carbon Dioxide CO2 Breath in O2 & breath out CO2 Hypoventilation leads to HIGHER CO2 Hyperventilation leads to lower CO2 Carbon diACID Hydrogen ions = HIGH acid KEY PLAYERS Kidneys control Acid Hydrogen H+ ions (acid) Found in the urine Bicarbonate HCO3 (base) Found in the intestines Base H⁺ O Hydrogen Acid O C O O C H O Bicarb Carbon dioxide Acid Metabolic Acidosis & Alkalosis - Causes Over 7.45 pH Metabolic ALKalosis Vomiting NGT suction Hypokalemia • Low K+ Potassium (below 3.5) • LOW K+ = AlKaLOWsis Compensation • Slow • Shallow respirations K < 3.5 LOW K+ AlKaLOWsis MEMORY TRICKS Under 7.35 pH Metabolic Acidosis Metabolic ALKalosis H⁺ H⁺ H⁺ H⁺ Diarrhea Renal Failure DKA - Diabetic Ketoacidosis Lactic AcidOSIS • Shock (low perfusion) • Sepsis (severe infection) Compensation • Rapid, deep respirations Memory tricks Memory tricks Base out the Butt Metabolic ACIDosis DKA - Diabetic Ketoacidosis Vomiting sounds like “ALKKK-alosis” Metabolic ACIDosis Diarrhea: if it comes out of your a$$idosis Renal Failure: when the kidneys fail, acid prevails!