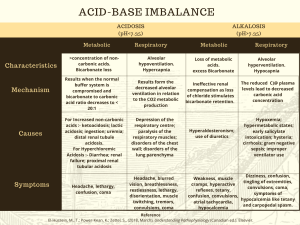
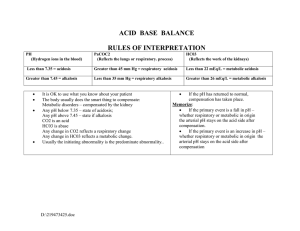
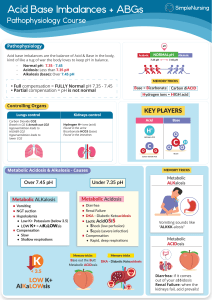
Acid Base Regulation in Surgery By Dr.Deepak Seshadri Ramarathnam M.D.Physician ( Stavropol State Medical University ) M.S ( General Surgery ) in Stavropol State medical university . Arterial blood gas (ABG) interpretation • in ALS (Advance life support) training , 5 steps approach to ABG interpretation is advocated . 1. How is the patient ? 2. Is the patient hypoxaemic ? The PaO2 on air should be 10.0-13.0 kPa. 3. Is the patient acidaemic (pH < 7.35 ) or Alkalaemic ( pH> 7.45 ) 4. What has happened to the PaCO2 ? If there is acidaemia , an elevated PaCO2 will account for this . 5. What is the Bicarbonate level or Base excess ? A metabolic acidosis will have a low bicarbonate level and a low base excess ( < -2mmol ) . A metabolic alkalosis will have a high bicarbonate and high base excess ( >+2mmol ). Respiratory failure Normal reference range • • • • • pH: 7.35 – 7.45 PaCO2: 4.7 – 6.0 kPa || 35 – 45 mmHg PaO2: 10 – 13 kPa || 80 – 100 mmHg HCO3–: 22 – 26 mEq/L Base excess (BE): -2 to +2 mmol/L Respiratory Acidosis • • • • • • • Rise in CO2 levels usually as a result of alveolar hypoventilation . Renal compensation may occur leading leading to compensated respiratory acidosis . CO2 level increases with pH levels decreases ( RO- Respiratory opposite ) Causes : 1) COPD 2) Decompression in other Respiratory conditions eg. Life threatening Asthma / Pulmonary Oedema . 3) Sedative drugs : Benzodiazepines , Opiate overdose . Respiratory alkalosis • Hyperventillation resulting excess loss of CO2 . This will result in increasing pH . ( RO - Respiratory opposite ) • Causes : 1. Psychogenic anxiety to hyperventilation 2.Hypoxia causing a subsequent hyperventilation : pulmonary embolism , high altitude . 3. Early salicylate poisoning . 4. CNS stimulation : Stroke , Subarachnoid hemorrhage , Encephalitis . 5. Pregnancy * Salicylate overdose leads to a mixed respiratory alkalosis and metabolic acidosis . Early stimulation of the respiratory centre leads to a respiratory alkalosis whilst later the direct acid effects of salicylates ( combined with Acute renal failure ) may lead to an acidosis . Metabolic acidosis • • • • • • • This is the most common surgical acid - base disorder Reduction in plasma bicarbonate levels Two mechanism : 1) Gain of strong acid ( eg. Diabetic Ketoacidosis ) . 2) Loss of base ( eg. from bowel in diarrhea ) . Classified according to the anion gap , This can be calculated by :(Na+K) -(Cl+HCO3). If a question supplies the chloride level , then this is often a clue that the anion gap should be calculated . Normal range = 8-16 mmol/L ( very few text books with 4-12 mmol/L). Normal Anion Gap (= hyperchloraemic metabolic acidosis ) : 1) GI bicarbonate loss : Diarrhea , Ureterosigmoidostomy , Fistula . 2) Renal tubular acidosis . 3) Drugs : Eg Acetazolamide . 4) Ammonium Chloride injection . 5) Addison’s disease . Raised anion Gap due to. 1) Lactate : Shock , Hypoxia.2) Ketones : Diabetic ketoacidosis , alcohol . 3) Urate : Renal failure . 4) Acid Poisoning : Salicylates , methanol . Metabolic acidosis secondary to high lactate levels may be subdivided into 2 types : 1).Lactic Acidosis type A ( Perfusion disorders eg. shock , hypoxia , burns ) . 2) Lactic acidosis type B ( Metabolic eg. Metformin toxicity ) . Metabolic Alkalosis • • • • • Usually caused by a rise in plasma bicarbonate levels . Rise in bicarbonate above 24 mmol/L will typically result in renal excretion of excess bicarbonate . Caused by loss of hydrogen ions or gain of bicarbonate . It’s due to mainly to problems of the kidney or Gastro intestinal tract . Causes : 1) Vomiting / aspiration (eg.Peptic ulcer leading to pyloric stenosis , nasogastric suction ) . 2) Diuretics . 3) Liquorice , Carbenoxolone . 4) Hypokalaemia . 5) Primary hyperaldosteronism. 6) Cushing syndrome . 7) Barter’s syndrome. 8) Congenital adrenal hyperplasia . Mechanism of metabolic alkalosis : 1) Activation of Renin II - aldosterone ( RAA) is a key factor . 2) Aldosterone causes reabsorbtion of Na+ in exchage for H+ in the distal convoluted tubule . 3)ECF depletion ( vomitting , diuretics ) resulting Na+ and cl- loss that results into activation of RAA system= raised aldosterone levels . 4) In Hypovolaemia,K+ shift from cells due to ECF , Alkalosis is caused by shift of H+ into cells to maintain neutrality . questions • 1) A 53 year old man is on the intensive care unit following an emergency abdominal aortic aneurysm repair. He develops • abdominal pain and diarrhoea and is profoundly unwell. His abdomen has no features of peritonism. Which of the • following arterial blood gas pictures is most likely to be present? • A. pH 7.45, pO2 10.1, pCO2 3.2, Base excess 0, Lactate 0 • B. pH 7.35, pO2 8.0, pCO2 5.2, Base excess 2, Lactate 1 • C. pH 7.20, pO2 9.0, pCO2 3.5, Base excess -10, Lactate 8 • D. pH 7.29, pO2 8.9, pCO2 5.9, Base excess -4, Lactate 3 • E. pH 7.30, pO2 9.2 pCO2 4.8, Base excess -2, lactate 1 • Answer: C • This man is likely to have a metabolic acidosis secondary to a mesenteric infarct. question • • • • • • 2) pH 7.36, PaCO2 7.3, PO2 8.9 (FiO2 40%), Bicarbonate 30.2, Base excess 5.3 Chronic respiratory acidosis with a compensatory metabolic alkalosis 3)pH 7.32, PCO2 3.8, PaO2 22.2 (FiO2 40%), Bicarbonate 19.1, Base excess -7.9 Metabolic acidosis with a compensatory respiratory alkalosis 4)pH 7.19, pCO2 10.2, pO2 16 (FiO2 85%), Bicarbonate 23.8, Base excess -2.2 mmol Acute respiratory acidosis. question • • • • • • • • • 5)A 67 year old male is admitted to the surgical unit with acute abdominal pain. He is found to have a right sided pneumonia. The nursing staff put him onto 15L O2 via a non rebreathe mask. After 30 minutes the patient is found moribund, sweaty and agitated by the nursing staff. An arterial blood gas reveals: pH:7.15; pCO2:10.2; pO2:8; Bicarbonate:32; Base excess:5.2. What is the most likely cause for this patients deterioration? A. Acute respiratory alkalosis secondary to hyperventilation B. Over administration of oxygen in a COPD patient C. Metabolic acidosis secondary to severe pancreatitis D. Metabolic alkalosis secondary to hypokalaemia E. Acute respiratory acidosis secondary to pneumonia • • Answer: B This patient has an acute respiratory acidosis, however this is on a background of chronic respiratory acidosis (due to COPD) with a compensatory metabolic alkalosis (the elevated bicarbonate is the main clue to the chronic nature of the respiratory acidosis). This blood gas picture is typical in a COPD patient who has received too much oxygen; these patients lose their hypoxic drive for respiration, therefore retain CO2 and subsequently hypoventilate leading to respiratory arrest. If the bicarbonate was normal, then the answer would be acute respiratory acidosis secondary to pneumonia. • • • • questions • • • • • A. pH 7.64 pO2 10.0 kPa pCO2 2.8 kPa HCO3 20 B. pH 7.25 pO2 8.9 pCO2 3.2 HCO3 10 C. pH 7.20 pO2 6.2 pCO2 8.2 HCO3 27 D. pH 7.60 pO2 8.2 pCO2 5.8 HCO3 40 E. pH7.45 pO2 7.2 pCO2 2.5 HCO3 24 • • • Pulmonary embolus The correct answer is pH7.45 pO2 7.2 pCO2 2.5 HCO3 24 A combination of hypoxia and respiratory alkalosis should suggest a pulmonary embolus. The respiratory alkalosis is due to hyperventilation associated with the pulmonary embolism. Ureterosigmoidostomy Answer: pH 7.25 pO2 8.9 pCO2 3.2 HCO3 10 There is acidosis. To compensate the patient will attempt to reduce the pH level in the blood by hyperventilating, hence the low CO2 level . Peptic ulcer causing pyloric stenosis Answer: pH 7.60 pO2 8.2 pCO2 5.8 HCO3 40 • • • • • • •