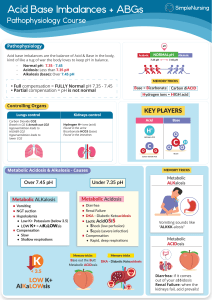
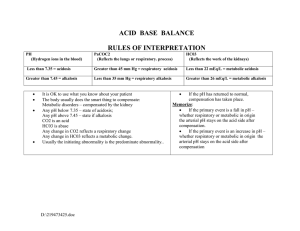
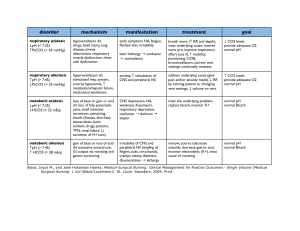
ACID BASE DISTURBANCES Hinkle and Cheever Chapter 10 Acid- base balance Homeostasis of the hydrogen ion concentration is Soooo important that the body is very creative to maintain balance Balance • Acid base is a balanced with a ratio of 1 carbonic acid to 20 parts bicarbonate base pH - Plasma pH is an indicator of hydrogen ion concentration: 7.35 - 7.45 Bases can accept hydrogen ions to convert strong acids to weak acids. • Primary base - bicarbonate HCO3• Lesser bases - hemoglobin, protein, and phosphate (less important buffer system) The body’s pH is maintained by: • Chemical buffering system • The lungs • The kidneys Three Regulatory Systems • Chemical Buffers – act immediately • Bicarbonate, Phosphate, and protein • Respiratory System – uses hypoventilation or hyperventilation as needed. • Kidneys – excretes or retains acids and bases as needed Chemical buffering • The body’s primary buffer system is: Bicarbonate (HCO3-) carbonic acid (H2CO3-) in the plasma Respiratory Regulation • When the amount of CO2 (carbon dioxide) produced is not equal to the amt removed, a CO2 imbalance occurs. • Chemoreceptor's in the medulla are sensitive to the concentration of CO2 and alter the rate and depth of respiration accordingly. • Either to remove – tachypnea or retain CO2 - bradypnea. • Carbonic acid - (H2CO3) Carbon dioxide and water combined. Most abundant body acid. • Respiratory compensation takes 12-24 hours to work Renal Regulation • Maximum response takes 3-4 days • Regulate bicarbonate (HCO3- Buffer) concentration in the ECF • Regenerate and reabsorb bicarbonate ions • In acidosis, the kidneys excrete hydrogen cations (H+) and reabsorb (conserve) bicarbonate anions (HCO3-). • To restore the balance with reabsorption of bicarbonate (HCO3-), Chloride anions (Cl-) are excreted • In alkalosis, the kidneys retain hydrogen ions and excrete bicarbonate ions and reabsorb (conserve) chloride anions. • Renal regulation is through Renal Regulation H+, Na+, HCO3- (bicarb). • H+ excreted into tubules, combined with HCO3, and forms H2CO3 (carbonic acid) • H2CO3 separates and forms H20 and CO2. • H2O is excreted and CO2 is converted back to HCO3 • In acidosis Lots of HCO3 is produced and in alkalosis little HCO3 is produced Na3HPO4 - Sodium phosphate is alkaline in water Serum Electrolytes • State of Acidosis: K+ shifts out of cell and H+ shifts into cell • State of alkalosis: K+ shifts into cell and H+ shifts out of cell • State of acidosis: Hco3- is conserved and cl- is excreted • State of alkalosis: Hco3- is excreted and cl- is conserved • Anion gap: difference between cations (Na+/K+) and anions (cl- and HCO3-) in blood. • The gap reflects unmeasured anions: phosphates, sulfates and proteins • >16 results from accumulation of fixed acids (Metabolic acids): lactate, ketoacids, uremia • Elevated anion gap is an indication of metabolic acidosis • Anion gap = Na+ - (Cl- + HCO3-) Normal 8 – 12 mEq/L • Anion gap = Na+ + K+ - (Cl- + HCO3-) Normal 12 – 16 mEq/L • The nurse reviews the following laboratory results • Na+ 145 • K+ 3.2 • Cl100 • HCO3- 24 • Calculate the anion gap • Anion gap = Na+ - (Cl- + HCO3-) • Is this metabolic or respiratory acidosis or alkalosis?? ABG pH 7.35-7.45 perfect 7.40 PaCO2 35-45 mm Hg HCO3 22-26 mEq/L PaO2 80-100 mm Hg <Hypoxemia Base Excess + or - 2 O2 saturation > 94% Respiratory Acidosis • Hypoventilation • Hypercapnia: PaCO2 >45mmHg • Acute: Drugs, cerebral injury, sudden cardiac arrest, Fx rib, airway obstruction, pneumonia, congestive heart failure • Chronic: COPD, cystic fibrosis, sleep apnea, kyphoscoliosis, brain tumor, obesity, ROME resp. acidosis Respiratory Acidosis - ROME pH No Compensation Partial Compensation Full Compensation pH 7.35 - 7.4 PaCO2 Lungs HCO3 Kidneys Respiratory Acidosis Signs and Symptoms Treatment • S/S: anxiety, headache, confusion, lethargy, cyanosis, Arrhythmias with elevated K+ • PaCO2 >60 -> cerebral vasodilation and edema • TX: Oxygen, antibiotics, bronchodilators, suction, reposition, chest physiotherapy, intubation, IV buffers and lowering K+ • (sodium bicarb) and ringers lactate IV • Treat cause Respiratory Alkalosis • Hyperventilation • Hypocapnia: PaCO2 <35 mm Hg • Stimulation of Respiratory Center: anxiety, fever, pain, salicylates, gram-neg sepsis, • Hypoxemia: pneumonia, high altitude, severe anemia, • Pulmonary disorders: pulmonary emboli, pulmonary edema, asthma (early) Resp alkalosis • S/S: Acute • Anxiety, • Lightheadedness: cerebral vasoconstriction • Paresthesia: (low Ca+) • Circumoral numbness • Dysrhythmias: (Low K+) • S/S: Severe • Confusion • Tetany • Syncope • Seizures • Treatment • Sedatives, O2, • Pain medication, IV.9NaCl • Treat Cause Respiratory Alkalosis - ROME pH No Compensation Partial Compensation Full Compensation pH 7.4 - 7.45 PaCO2 Lungs HCO3 Kidneys Metabolic Acidosis • HCO3- <22 mEq/L • With acidemia: pH <7.35 • Cause • Loss of Base: diarrhea, ileostomy, renal failure with decrease HC03- production, acetazolamide –Diamox (carbonic anhydrase inhibitor) CO2 + H2O <--CA--> H2CO3 <--> H+ + HCO3-. Diuretic leading to excretion of base • Excess Acid Production: ketoacidosis, alcohol & starvation, DKA, lactic acidosis with anaerobic metabolism (drop in B/P), • Excess Acid Ingestion: salicylates, cocaine, ecstasy, methamphetamine • Inability of kidney to excrete H+ ions: renal failure, K+ sparing diuretics Metabolic acidosis • S/S • Headache • Confusion drowsiness • N & V, diarrhea • Vasodilation: low B/P, tachycardia, tachypnea • Severe • Dehydration: s/s • Dysrhythmias: hyperkalemia • Kussmaul’s-rapid/deep compensation • Stupor, coma • Treatment • Sodium bicarbonate (NaHCO3) or lactate ringers • Correct underlying disease: dialysis, Insulin, fluids, glucose • Check anion gap • Cause: Pancreatitis, renal or liver failure, DM, dehydration, diarrhea, ETOH, ASA, diuretics Metabolic Acidosis - ROME pH No Compensation Partial Compensation Full Compensation pH 7.35 - 7.4 PaCO2 Lungs HCO3 Kidneys Metabolic Alkalosis • HCO3- > 26 mEq/L • With alkalosis pH >7.45 • H+ ion loss: N/V, NG sx, • H+ shift out of the cells: hypokalemia, rapid correction post starvation and Hi PCO2 levels when HCO3- is still elevated • Hypokalemia: K+ moves out of Vessels and H+ moves into Vessel to correct • Bicarbonate retention: bicarbonate antacids intake PO IV or citrate in blood transfusions. • Contraction Alkalosis: diuretics (loss of fluids with H+, Cl-, and HCO3-) HCTZ & lasix Metabolic Alkalosis • S/S: Many d/T low K+/Ca++ • Paresthesia: (low Ca) • Dizziness • Muscle weakness (low K+) • Hyporeflexia (Low K+) • Dysrhythmia (Low K+) • Bradypnea: resp compensation • Severe: • Tetany : (low Ca) • Confusion • stupor • Treatment • Correct underlying disease: • Urine loss of Cl-: stop diuretics • Low K+: IV KCL • Isotonic fluid tx diuretic effect • Acetazolamide: Diamox (carabonic Anhydrase inhibitor) blocks HCO3- reabsorption) Metabolic Alkalosis - ROME pH No Compensation Partial Compensation Full Compensation pH 7.4 - 7.45 PaCO2 Lungs HCO3 Kidneys Elderly • High risk group • Lots of meds: Diuretics • Poor diet, low Na, • Co-morbidities: renal, cardiac, pulmonary, endocrine, • Compensation slow!!! • Risk of falls enhanced with weakness • Mrs. R.L., a 68-year-old woman, is being seen in the emergency department after 3 days of diarrhea. Her son says that his mother seemed confused and drowsy for the past 2 days. Mrs. R.L. receives dialysis treatment three times a week, her last treatment being yesterday. On admission the patient’s vital signs are: • blood pressure, 108/56 mm Hg; respiration, 28 breaths/min (regular, deep and rapid); pulse, 98 beats/min; and temperature, 37º C (98.9º F). • The results of the arterial blood gases on Mrs. R.L. are: • pH - 7.32; HCO3 - 20; and PaCO2 - 40. • What acid-base imbalance does this patient have? • What are the s/s of this acid base imbalance? • What are some interventions that can help this patient? • What caused Mrs. R.L. to have this acid-base imbalance? • A patient has a 3 day history of nausea and vomiting. ABG results are: • pH 7.51 • PaCO2 42 mm Hg • HCO3 34 mEq/L • PaO2 92 • What acid base disorder is this acidemia or alkalemia? • Is it respiratory or metabolic? • Has compensation occurred? • What contributed to the condition? • What s/s would you assess for? • What treatment is indicated?