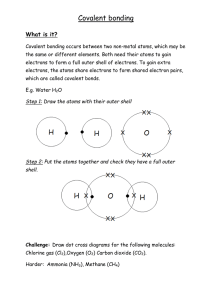
Knowledge Organiser for Unit 1: Particulate Nature of Matter Solid Liquid The particles in a solid are arranged in a regular pattern. The particles in a solid vibrate in a fixed position and are tightly packed together. The particles in a solid have a low amount of kinetic energy. The particles in a liquid are randomly arranged. The particles in a liquid are able to move around each other. The particles in a liquid have a higher amount of kinetic energy than particles in a solid. Solids have a fixed shape and are unable to flow like liquids. The particles cannot be compressed because the particles are very close together. Liquids are able to flow and can take the shape of the container that they are placed in. As with a solid, liquids cannot be compressed because the particles are close together. Phase Changes The three states of matter are solid, liquid, and gas. For a substance to change from one state to another, energy must be transferred. The particles gain energy. This results in the breaking of some of the attractive forces between particles during melting. Phase changes are physical changes because there are no new substances formed. For example, when ice (water in solid state) melts, it is still water, only in the liquid state. To evaporate or boil a liquid, more energy is needed to overcome the remaining chemical bonds between the particles. Note the difference between boiling and evaporation. When a liquid evaporates, particles leave the surface of the liquid only. When a liquid boils, bubbles of gas form throughout the liquid before rising to the surface and escaping. Gas The particles in a gas are randomly arranged. The particles in a gas are able to move around very quickly in all directions. Of the three states of matter, gas particles have the highest amount of kinetic energy. Gases, like liquids, are able to flow and can fill the container that they are placed in. The particles in a gas are far apart from one another which allows the particles to move in any direction. Gases can be compressed because they have a large space between the particles. When squashed, the particles have empty space to move into. Identifying the Physical State & Diffusion If the given temperature of a substance is lower than the melting point, the physical state of the substance will be solid. If the given temperature of the substance is between the melting point and boiling point, the substance will be a liquid. The amount of energy needed for a substance to change state is If the given temperature of the substance is higher than the boiling dependent upon the strength of the attractive forces between point, the substance will be a gas. particles. The stronger the forces of attraction, the more energy is needed to break them apart. Substances that have strong attractive Diffusion forces between particles generally have higher melting and boiling • movement of a substance from a region of higher points. concentration to a region of lower concentration • lighter substances travel faster Melting, vaporization, and sublimation are endothermic processes, • increasing temperature would make diffusion faster whereas freezing, condensation, and deposition are exothermic processes. Knowledge Organiser for Unit 2: Experimental Techniques Pure Substances Chromatography Pure substances, in chemistry, only contain one type of element or one type of compound. For example, pure water will just contain water (a compound). Paper chromatography is a separation technique that is used to separate mixtures of soluble substances. How soluble a substance is determined how far it will travel across the paper. In our everyday language, we use the word ‘pure’ differently from how it is used in chemistry. Pure can mean a substance that has had nothing else added to it and is in its natural state. An example of this is pure orange juice. This means that the bottle will just contain orange juice and no other substances. Pencil is used to draw the baseline because ink dissolves in the solvent; the ink spot should also be above the solvent. Elements are made up of one type of atom. It is the simplest pure substance. For example, oxygen is made up of oxygen atoms. Carbon is made up of carbon atoms. Compounds are two or more elements that are chemically joined together.For example, NaCl which is sodium chloride. Mixtures are two or more pure substances (elements or compounds) that are NOT chemically joined together. An example of this is a standard cup of coffee. Coffee contains water, milk, coffee, and possibly sugar. The components of the cup of coffee are not bonded together. Pure substances have a sharp melting point compared to impure substances which melt over a range of temperatures. Impurities lower the melting point of a substance while its boiling point increases. The separation of the dissolved substances produces what is called a chromatogram. Rf Value Different compounds have different Rf values in different solvents. The Rf values of known compounds can be used to help identify unknown compounds. The higher the Rf value, the farther the solute travelled with the solvent. Hence, the more attraction it has with the solvent. In paper chromatography, this can be used to distinguish between those substances that are pure and those that are impure. Pure substances have one spot on a chromatogram as they are made from a single substance. Impure substances produce two or more spots as they contain multiple substances. By calculating the Rf values for each of the spots, it is possible to identify the unknown substances. Similarly, if an unknown substance produces the same number and colour of spots, it is possible to match it to a known substance. Chromatography can be used also in colourless substances. A locating agent is a substance used for detecting colourless substances. Take a look at the sample above. To compute the Rf value of the substance from green, you will compute it this way: Rf value = 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑡𝑟𝑎𝑣𝑒𝑙𝑙𝑒𝑑 𝑜𝑓 𝑠𝑢𝑏𝑠𝑡𝑎𝑛𝑐𝑒 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑡𝑟𝑎𝑣𝑒𝑙𝑙𝑒𝑑 𝑏𝑦 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 8𝑐𝑚 Rf value = 10 𝑐𝑚 = 0.8 Rf value will always have a value of less than 1. Cont. Unit 2: Experimental Techniques Mixtures – in a mixture there are no chemical bonds, so the substances are easy to separate by physical means. Examples of mixtures are air and salt water. The method for separating the mixture is based on the following factors: • physical state of the mixture • the substance that will be collected • properties of substances Distillation - To separate out mixtures of miscible liquids; the mixture is heated to let the substance with a lower boiling point evaporate first, leaving the other substance in the distilling flask; the vapour will turn back to liquid using the condenser a. Simple distillation – separating a liquid from a solution b. Fractional distillation – separating out a mixture of liquids; can be used to separate crude oil into fractions Condenser Condenser Fractionating column Other Methods of Separating Mixtures Evaporation – to separate a soluble salt from a solution; a quick way of separating out the salt. Crystallisation – to separate a soluble salt from a solution; a slower way of separating out the salt. Separating out a soluble salt from insoluble salt 1. Add water and stir the substances until the soluble salt dissolves. 2. Filter the mixture, leaving the insoluble substance in the filter paper. 3. Evaporate the water from the salt, leaving the crystals. Filtration – to separate an insoluble solid from a liquid; this method will make the cloudy mixture into a clear and transparent liquid filtrate. Solid insoluble substances that cannot pass through the filter paper is called the residue. Filtered liquid is called a filtrate. Distilling flask Common Laboratory Apparatus for Chemistry Practical Investigations conical flask funnel pipette - for stirring or heating relatively large amounts of liquids; collection flask for titration - for filtration to contain the filter paper - for transporting accurate volume of liquids (e.g. acids) burette measuring cylinder thermometer - for dispensing accurate volumes of liquids; to see changes in volume for titration - for measuring the accurate volume of a liquid or solutions - for measuring temperature Knowledge Organiser for Unit 3: Atoms, Elements, Compounds Atomic Structure Electronic Structure of Elements Atoms Contained in the nucleus are the protons and neutrons. Moving around the nucleus are the electron shells. Below are the charges and relative mass of the three subatomic particles. An atom has an atomic nucleus with a (+) charge at the centre, and electrons with a negative charge around it. Electrons are arranged according to certain rules that a maximum number of electrons can occupy each shell. Once it’s occupied, the next electrons will fill up the next shells. Rule for the first 20 elements: • First shell (energy level) contains a maximum of 2 electrons • Second shell can hold a maximum of 8 electrons • Third shell can hold a maximum of 8 electrons Overall, atoms have no charge; they have the same number of protons as electrons. An ion is a charged particle - it does not have an equal number of protons to electrons. Mass number Atomic Number and Mass Number (Nucleon Number) Atomic number = number of Protons = number of Electrons Mass number = number of Protons + number of Neutrons Mass number - Atomic number = number of Neutrons Atomic number X Charge Subscript 𝟏𝟐 𝟔𝐂 Writing the electronic structure of the element: • Identify the atomic number of the element. • Distribute the electrons per shell of the element. • Write the number of electrons per shell and separate with a comma. Electron structure of the first 20 elements Elements and Compounds Elements are made of atoms with the same atomic number. They are in the periodic table. Elements cannot be separated into simpler parts by either physical or chemical processes. Atoms are represented by symbols. N = nitrogen F = fluorine Zn = zinc Ca = calcium Isotopes – an isotope is an element with the same number of protons but a different number of neutrons. They have the same atomic number, but a different mass number. Compounds – a compound is when two or more elements are chemically joined. Examples of compounds are carbon dioxide and magnesium oxide. Some examples of formulas are CO2, NaCl, HCl, H2O, and Na2SO4. They are held together by chemical bonds and are separated ONLY by chemical means. When you add the electrons at the bottom of the name of element, it will add up to its atomic number. The valence electron is the Valence last number in the electronic electron structure of the element because it is found in the outermost shell. The number of valence electron of the element is equal to its group number in the periodic table. Knowledge Organiser for Unit 3: Atoms, Elements, Compounds Formation of Ions Ionic Bonding Ions are charged particles. They can be either Ionic bonding occurs between a metal and a non-metal. Metals positively or negatively charged, for lose electrons to become positively charged. Opposite charges example, Na+ or Cl-. are attracted by electrostatic forces – an ionic bond. When an element loses or gains electrons, it becomes an ion. Non-metals gain electrons to become negatively charged, called anions. Group 1 and 2 elements lose electrons and group 6 and 7 elements gain electrons. Ions +1 +2 -2 -1 Element Example Li → Li+ + eCa → Ca2+ + 2eBr + e- → BrO + 2e- → O2- Ionic Compounds Ionic compounds, also called salts, form structures called giant lattices. There are strong electrostatic forces of attraction that act in all directions and act between the oppositely charged ions that make up the giant ionic lattice. Metals and Non-metals Metals are found on the left-hand side of the periodic table. Metals are strong, shiny, malleable, and good conductors of heat and electricity. On the other hand, non-metals are brittle, dull, not always solids at room temperature, and poor conductors of heat and electricity. Non-metals are found on the right-hand side of the periodic table. Covalent bonding is the sharing of a pair of electrons between atoms to gain a full outer shell. This occurs between two nonmetals. Metalloids form this bond with a non-metal element. Compounds created by covalent bonding are called covalent compounds or molecules. Simple covalent bonding occurs between the molecules below. Dot-and-cross diagrams are useful to show the bonding in simple molecules. The outer electron shell of each atom is represented as a circle, the circles from each atom overlap to show where there is a covalent bond, and the electrons from each atom are either drawn as dots or crosses. There are two different types of dot and cross diagrams – one with a circle to represent the outer electron shell and one without. Metals lose electrons to become positively charged, called cations. Group 1 2 6 7 Covalent Bonding Properties of Simple Covalent Compounds • Low melting and Low boiling points – this is because the weak intermolecular forces that hold the molecules together break when a substance is heated, not the strong covalent bonds between atoms • Do not conduct electricity – as they do not have any free delocalized (mobile) electrons Example Dot-and-Cross Diagram of Covalent Compounds Properties of Ionic Compounds • High melting point and High boiling point – lots of energy is needed to overcome the electrostatic forces of attraction • When molten or in solution, can conduct electricity – as the ions are free to move & can carry the electrical current • Cannot conduct electricity in a solid – as the ions are not free to move NH3 (ammonia) CH4 (methane) Unit 3: Atoms, Elements, Compounds Covalent Bonding of Simple Molecules The following simple covalent structures are for hydrogen, oxygen, chlorine, methane, hydrogen chloride, and water. H2 (Hydrogen molecule) Giant Covalent Structure - Diamond Giant Covalent Structure - Graphite Each carbon atom is bonded to four other carbon atoms, making the diamond very strong. Diamond has a high melting and boiling point. Graphite is made up of layers of carbon arranged in hexagons. Each carbon is bonded to three other carbons. Large amounts of energy are needed to break the strong covalent bonds between each carbon atom. Diamond does NOT conduct electricity because it has no free electrons. Graphite has one free delocalized electron that is able to move between the layers which is why it can conduct electricity. The layers are held together by weak intermolecular forces. The layers of carbon can slide over each other easily which is why it can be a lubricant. Cl2 (Chlorine molecule) O2 (Oxygen molecule) Graphite has a high melting point because a lot of energy is needed to break the covalent bonds between the carbon atoms. Giant Covalent Structure – Silicon Dioxide Silicon dioxide (silicon and oxygen atoms) has a similar structure to that of diamond, in that its atoms are held together by strong covalent bonds. HCl (Hydrogen chloride) Large amounts of energy are needed to break the strong covalent bonds therefore silicon dioxide, like a diamond, has a high melting and boiling point. H2O (water molecule) The Modern Periodic Table Elements are in order of increasing atomic mass/proton number. It shows where the metals, metalloids, and nonmetals are. Metals are on the left (red color), metalloids are the ‘staircase’ in yellow, and non-metals are on the rightmost in green. The columns show the groups. The group number shows the number of electrons in the outer shell. The rows are periods – each period shows another full shell of electrons. The periodic table can be used to predict the reactivity of elements.