Mater, Energy, Elements, and Atoms
advertisement

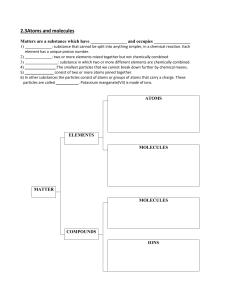
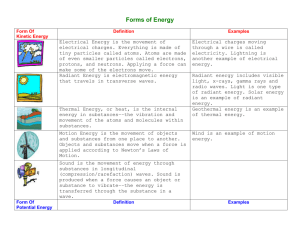
Matter- Anything that occupies soace and has mass Energy- the ability to do work Elements- any of the building blocks of matter, oxygen, hydrogen, carbon, for example Atoms- the smallest part of an element: invisible by ordinary chemical means Chemical- is stored in the bond of chemical substances Electrical- results from the movement of charged particles. Mechanical- is directly involved in moving matter Radiant- travels in waves, that is energy of the electromagnetic spectrum All mater is composed of these limited substances called elements, they're unique substances that can not be broken down into simpler substances by ordinary chemical methods. The word atom comes from the Greek word meaning “ incapable of being divided,” and historically this idea of was accepted as truth. We now know that atoms, although tiny, are clusters of subatomic particles and that, under special circumstances that atoms can be split into these smaller particles which are neutrons, electrons, and protons. Matter is the “stuff” of the universe. With some exceptions, it can be seen, smelled, and felt. More accurately, matter is anything that occupies space and has mass. Matter can be changed physically and chemically. Physical changes don’t alter the basic nature of the substance, while chemical alters the composition of the substance. Chemical Changes- Burning wood, fermenting grapes, digesting food Physical- Crumpling paper, melting ice, cutting food into smaller pieces https://www.youtube.com/watch?v=nW hzeJoEVBA Marieb, Elaine. “Matter, Energy, Elements, and Atoms” Essentials of Human Anatomy and Physiology. Serina Beauparlant. 8th Edition. San Francisco: Pearson Benjamin Cummings, 2006. Pages 26-29: table 2.1 included. Print