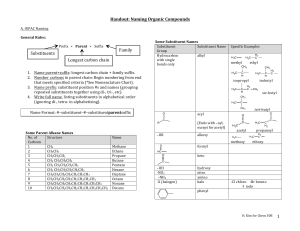
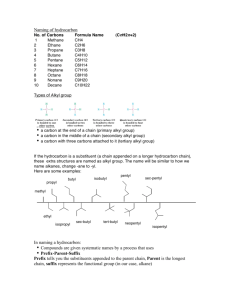
Handout: Naming Organic Compounds A. IUPAC Naming General Rules: Prefix + Parent + Suffix Substituents Family Longest carbon chain Some Substituent Names Substituent Substituent Name Group Hydrocarbon alkyl with single bonds only 1. Name parent+suffix: longest carbon chain + family suffix. 2. Number carbons in parent chain: Begin numbering from end that meets specified criteria (*See Nomenclature Chart). 3. Name prefix: substituent position #s and names (grouping repeated substituents together using di-, tri-, etc). 4. Write full name, listing substituents in alphabetical order (ignoring di-, tetra- in alphabetizing). Methane Ethane Propane Butane Pentane Hexane Heptane Octane Nonane Decane H2 C H 3C methyl ethyl CH 3 CH 3 C H H 3C isopropyl H 3C C H C H H2 C isobutyl H2 C CH 3 sec-butyl CH 3 H 3C C CH 3 O C Name H 3C H 3C Name Format: #–substituent–#–substituentparentsuffix Some Parent Alkane Names No. of Structure Carbons 1 CH4 2 CH3CH3 3 CH3CH2CH3 4 CH3 CH2CH2CH3 5 CH3 CH2CH2CH2CH3 6 CH3 CH2CH2CH2CH2CH3 7 CH3CH2CH2CH2CH2CH2CH3 8 CH3CH2CH2CH2CH2CH2CH2CH3 9 CH3CH2CH2CH2CH2CH2CH2CH2CH3 10 CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 Specific Examples acyl R (Ends with –oyl, except for acetyl) –OR alkoxy O formyl tert-butyl O O C C H 3C H 3C acetyl propanoyl H 3C H 3C methoxy C H2 H2 C ethoxy HC O keto C –OH -NO2 –NH2 -X (halogen) hydroxy nitro amino halo -Cl chloro -Br bromo -I iodo phenyl H. Kim for Chem 30B 1 B. Common Names Some Widely-Used Common Names Compound Common Name CH2=CH2 ethylene CH3CH=CH2 propylene HC CH acetylene O acetone C H 3C IUPAC Name 1-ethene 1-propene 1-ethyne 2-propanone CH 3 O formaldehyde methanal acetaldehyde ethanal formic acid methanoic acid acetic acid ethanoic acid propionic acid propanoic acid butyric acid butanoic acid CH2 O CH H 3C O HC OH O H 3C C OH O H 3C C H2 C OH O H 3C H2 C C H2 C OH H. Kim for Chem 30B 2 Nomenclature Chart for Organic Compounds (From HIGHEST TO LOWEST naming priority) FAMILY Carboxylic acid Ester Amides Aldehydes Ketones Alcohol PARENT Parent is longest carbon chain containing carboxylic acid. Derive name from parent alkane. Name of alkyl group that replaced -H in -COOH + name derived from parent acid, replacing –ic acid with –ate Derive name from parent carboxylic acid, replacing –oic acid with –amide. Derive name from parent alkane. SUFFIX –oic acid (-dioic acid for dicarboxylic acid; -enoic acid for unsaturated acid) –ate –amide NUMBERING Begin at carbonyl C. NOTES Common names are often used (C next to COOH group is designated as “α”). EXAMPLES [Salts: cation + name derived from parent acid, replacing –ic acid with –ate ] 2-chloropropanoic acid or α-chloropropionic acid (common) O H 3C H C C OH Cl Begin at carbonyl C. Begin at carbonyl C. Common names are often used: name of alkyl group that replaced -H in -COOH + name derived from common name of parent acid, replacing –ic acid with –ate Alkyl substituents on nitrogen start with “N-.” O H 3C H2 C H2 C C O H2 C CH 3 ethyl butanoate or ethyl butyrate (common) H 3C O CH 3 C N CH 3 N,N-dimethylacetamide –al Derive name from parent alkane. Parent name starts with position # of carbonyl C. –one Parent is longest carbon chain containing OH. Parent name starts with position # of the C with OH. –ol (-diol, -triol, etc.) Begin at carbonyl C. Begin at end nearer to carbonyl C. Begin at end nearer to OH group. Common names are often used for simplest aldehydes, ending with “–aldehyde.” Common names are often used for simple ketones: names of two alkyl groups + “ketone.” Cyclic alcohols: Parent name begins with “cyclo” (no need to start parent name with “1”). Begin numbering at C with OH, and number to give substituents lowest numbers. CH 3 H 3C C H O H2 C C H 3-methylbutanal or β-methylbutyraldehyde (common) O H 3C H2 C H2 C C CH 3 2-pentanone or methyl propyl ketone (common) CH 3 H 3C C H OH H2 C H2 C C H CH 3 5-methyl-3-hexanol OH CH 3 2-methylcyclohexanol OH H 3C Thiols –thiol Name in same way as alcohols, except end with “-thiol.” H 3C C H H2 C H2 C SH H2 C OH 1,3-butanediol ethanethiol H. Kim for Chem 30B 3 FAMILY Amines Alkenes Alkynes Alkanes Ethers PARENT SUFFIX –amine Parent is longest carbon chain containing the double or triple bond. Parent name starts with position number of multiple bond. May need cis/trans designation. –ene –yne Parent is longest carbon chain. –ane The alkoxy group –OR is treated as the substituent (Alkane or another functional group is the parent). (-diene, -triene, etc.) NUMBERING Begin at end closer to multiple bond. (If multiple bonds are equidistant, give smaller number to first branch point). Then give smallest numbers possible to substituents. Begin at end nearer to branch point. Then give smallest numbers possible to substituents. NOTES 1° amines, and 2°, 3° amines with same R groups on N: Treat alkyl groups attached to nitrogen as substituents. For same substituents, use “di” and “tri.” 2°, 3° amines with different R groups on N: Parent amine is the one with largest R group; name other groups as substituents, starting with N-. [Ions derived from amines: Replace –amine with –ammonium.] Cyclic alkenes: Parent name begins with “cyclo” (no need to start parent name with “1”). Number multiple bonds 1 and 2, in direction to give first substituent the next smaller possible number. EXAMPLES H2 C H 3C H2 C NH 2 (1°) propylamine H 3C H2 C H2 C N H 2C (3° with same R groups) CH 3 CH 3 triethylamine H2 C H 3C H2 C H N CH 3 (2° with diff’t R groups) N-methylpropanamine H 3C H2 C CH2CH2CH 3 C C H CH 3 cis-4-methyl-3-heptene H 3C H2 C H2 C H2 C C C CH 3 2-heptyne CH 3 4-methylcyclohexene Cyclic alkanes: Parent name begins with “cyclo.” Give smallest number to substituent that comes first in alphabetical order. Number in direction to give second substituent the smaller possible number. (If single substituent, don’t need “1-.”) Common names are often used for simple ethers: two R groups + “ether.” Common names are used for cyclic ether compounds. CH 3 H 3C C H H2 C H2 C H C H 2C CH 3 CH 3 4-ethyl-2-methylhexane H2 C H 3C CH 3 1-ethyl-3-methylcyclohexane H 3C H2 C O H2 C H2 C CH 3 1-ethoxypropane or ethyl propyl ether (common) H 3C H2 C O H2 H2 H2 C C C OH 3-ethoxypropanol H. Kim for Chem 30B 4 Haloalkanes (or Alkyl Halides) Halogen atom is treated as substituent (Alkane or another functional group is the parent). Common names are often used, in format “alkyl halide.” Aromatic Nomenclature (*Functional group priority is same in aromatic and aliphatic nomenclature.) FAMILY PARENT SUFFIX NUMBERING NOTES Benzene “benzene” or common For di-substituted Common names for substituted name for substituted benzenes, o, m, p benzenes are often used benzene typically used for (accepted by IUPAC): CH 3 positions of OH substituents. o- Toluene Phenol H 3C H2 C Br 1-bromoethane or ethyl bromide (common) EXAMPLES NO 2 Cl m-chloronitrobenzene OH O O CH C OH O2N p-nitrophenol mBenzaldehyde Benzoic acid NH 2 pAniline H. Kim for Chem 30B 5