5-2 R/S System
advertisement

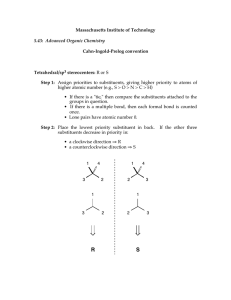
Stereocenters are labeled R or S. The convention for naming enantiomers unambiguously was developed by R.S. Cahn, C. Ingold, and V. Prelog. The four substituents around the chiral carbon must be first ranked in order of decreasing priority. •a highest priority •b second-highest priority •c third-highest priority •d lowest priority When the molecule is positioned with the lowest-priority substituent away from the viewer, the remaining three substituents will be arranged in either a clockwise or counterclockwise direction. If the progression from a to b to c is clockwise, the configuration at the stereocenter is named S (sinister) otherwise the configuration is named R (rectus) The R or S is added as a prefix in parentheses to the name of the chiral compound. (R)-2-bromobutane (S)-2,3-dihydroxypropanal (R,S)-bromochlorofluoromethane (a racemic mixture) If known, the sign of rotation of plane-polarized light may also be added, however, there is no correlation between R,S and +,-: (R)-(+)-2,3-dihydroxypropanal. Sequence rules assign priorities to substituents. Rule 1: Look first at the atoms directly attached to the stereocenter. Precedence is in order of highest atomic number. Hydrogen is always the lowest precedence. A higher-mass isotope takes precedence over a lower-mass isotope. Rule 2: If two atoms are of the same precedence using rule 1, proceed along the two respective substituent chains until you reach a point of difference. Verify these two examples: Rule 3: Double and triple bonds are treated as if they are single and the atoms in them are duplicated or triplicated at each end by the respective atoms at the other end of the multiple bond. Verify these assignments: