Document 10566232
advertisement

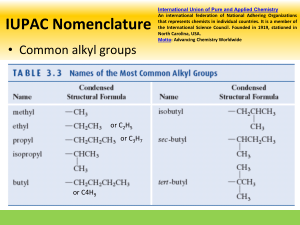
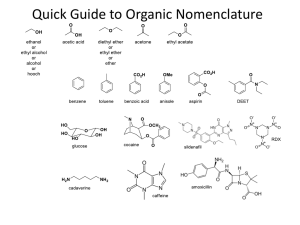
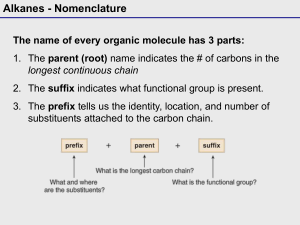
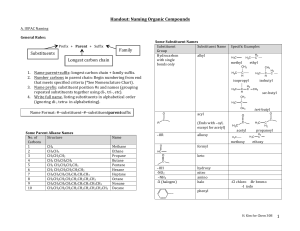
A note addressed to the teacher specifically explaining absence from a test or major assignment may be required Unexplained absences on “drop dead” deadline dates or test days will adversely affect a student’s marks The school as a whole is promoting Respect, Responsibility and Success within the school community Interim report cards will th be issued on March 8 and we look forward to seeing you on Parents’ nd Night, March 22 You can go to the science department response site at http://www.ugdsb.on.ca/cddhs/f orm.aspx?ekfrm=20249 to submit information directly. Completing the summative assignment is a requirement of demonstrating mastery of the overall objectives of every science course in order to earn a credit. Students are responsible for providing evidence of their learning within established timelines. A pattern of not completing work or submitting work late will jeopardize the granting of a credit If a student is legitimately absent ... it will be the student’s responsibility, on the first class day he or she returns to school, to submit the assignment or make arrangements to write a similar test for credit If a student is present at school for part of the day of an assignment deadline, the student will submit the assignment before leaving from or after returning to school on the assigned day Directly or indirectly using the work of others as original work is a serious offense and will result in academic penalties Students are responsible for returning all assigned texts and materials in their original condition. Any materials not returned, or damaged while assigned, will be replaced by the student. Textbook replacement costs range from $80 to $110. Safety is a critical part of the school science program. Students will be instructed in safe practices and will be expected to conduct all lab work in the safest manner. Safety First! Safety Contract Organic Compounds · Contain carbon · Have covalent bonds · Have low melting points · Have low boiling points · Burn in air (oxygen) · Are soluble in nonpolar solvents · Form large molecules Alkanes ¢ Contain C and H only ¢ Contain single bonds C-C ¢ Have ¢ Are 4 bonds to every carbon (C) atom nonpolar t Shapes of Alkanes “Straight-chain” alkanes have a zig-zag orientation when they are in their most straight orientation H Straight chain alkanes are also called unbranched alkanes l Nomenclature of Unbranched Alkanes l Nomenclature of Branched -Chain Alkanes (IUPAC) Locate the longest continuous chain of carbons; this is the pare chain and determines the parent name. Number the longest chain beginning with the end of the chain nearer the substituent Designate the location of the substituent When two or more substituents are present, give each substituent a number corresponding to its location on the longest chain H Substituents are listed alphabetically nt When two or more substituents are identical, use the prefixes tri- , tetraH H etc. Commas are used to separate numbers from each other The prefixes are used in alphabetical prioritization When two chains of equal length compete to be parent, choose the chain with the greatest number of substituents When branching first occurs at an equal distance from either end of the parent chain, choose the name that gives the lower number at the first point of difference di-, When two or more substituents are identical, use the prefixes tri-, tetraH H etc. Commas are used to separate numbers from each other The prefixes are used in alphabetical prioritization When two chains of equal length compete to be parent, choose the chain with the greatest number of substituents When branching first occurs at an equal distance from either end of the parent chain, choose the name that gives the lower number at the first point of difference di-, l Nomenclature of Branched Alkyl Chains Two alkyl groups can be derived from propane Four groups can be derived from the butane isomers The neopentyl group is a common branched alkyl group Examples The number of constitutional isomers possible for a given molecular formula increases rapidly with the number of carbons Father of organic chemistry synthesized urea Heidelberg 1828 The most important hypothesis in all of biology…is that everything that animals do, atoms do. In other words, there is nothing that living things do that cannot be understood from the point of view that they are made of atoms acting according to the laws of physics.” Richard Feynman (1918-1988)