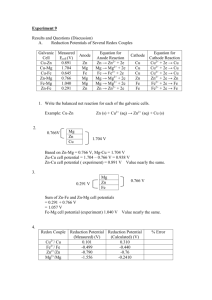
C.T PILLAY 21910221 GROUP 3A M SABELA 5TH SEPTEMBER 2019 Contents Introduction ............................................................................................................................................ 2 Methodology........................................................................................................................................... 3 Apparatus............................................................................................................................................ 3 Reagents ............................................................................................................................................. 3 Procedure ............................................................................................................................................ 3 Instrument parameters ....................................................................................................................... 4 Calculations ............................................................................................................................................. 4 Table 1: Calculation of masses to be used .......................................................................................... 4 Table 2: Calculation for equal quantities of the copper solution to be used ...................................... 4 Table 3: Separate calculations ............................................................................................................ 5 Results ..................................................................................................................................................... 6 Table 4: Measured results ................................................................................................................... 6 Table 5: Calculated results .................................................................................................................. 6 Table 6: Required information for the graph below ........................................................................... 7 Graph 1: A graph comparing Ecell and log[Cu2+] for cells 7,8,9 and 10. ............................................... 7 Discussion................................................................................................................................................ 8 Conclusion ............................................................................................................................................... 8 Safety/Precautions .................................................................................................................................. 9 References .............................................................................................................................................. 9 1 Introduction The aim of this experiment is to determine the electrode potentials of some metal electrodes. Electrochemistry is a branch in chemistry that deals with the interconversion of electrical energy and chemical energy. There are two types of cells. A galvanic or voltaic cell, which converts chemical energy to electrical energy and an electrolytic cell, which converts electrical energy to chemical energy. Electrochemical cells contain two electrodes, namely the anode (where oxidation takes place) and the cathode (where reduction takes place), these are the basis for this process. The metal electrodes must be placed in an electrolyte containing the same type of metal that makes up the electrodes. When the metal reacts, they give away electrons and form positive ions. The electrons are transferred through an external circuit, by an electromotive force, while ions are transferred through a salt bridge. The salt bridge maintains the overall charge balance for the two compartments. The potential that develops in a cell is the measure of the tendency for a reaction to proceed toward equilibrium. E0 values give you a way of comparing the positions at equilibrium, as well as predicting if it is spontaneous or not. 2 Methodology Apparatus A Balance 50 ml volumetric flasks 100 ml beakers Funnels Plastic droppers Pipettes Pipette pump Small plastic dish Copper, Zinc, Iron and Lead electrodes Digital Multimeter Salt bridge Reagents Copper(II) nitrate Zinc nitrate Lead nitrate Ammonium iron(II) sulphate Procedure The mass that was needed to prepare a 1 molar concentration of each reagent was calculated. The empty plastic dish was weighed and recorded. The required mass of each reagent was then weighed. Each reagent was transferred into separate 50 ml volumetric flasks using a funnel, ensuring the stem does not get clogged. The funnel was removed, and each sample was diluted to the calibration mark with deionised water. A stopper was added to the flasks and then swirled till the reagent was dissolved. Each flask was labelled. By serial dilutions, four copper(II) solutions of concentrations 0.1M, 0.01M, 0.001M and 0.05M were prepared in 50 ml volumetric flasks, using the 1M copper solution that was previously prepared. The flasks were then filled with deionised water to the calibration mark, and then swirled, to evenly dilute it. The flasks were labelled accordingly. Equal quantities of each of the metal ion solutions were calculated and then poured into four different 100 ml labelled beakers. The potentials were obtained, using a Digital Multimeter. The zero on the Multimeter was checked by short-circuiting across the positive and negative leads. The leads were then connected to the metal electrodes, so that a positive reading was displayed. A salt bridge taken from a solution was also connected to each half cell. The highest potential shown in the first 30 seconds was recorded, as well as the electrode which produces a positive reading. The procedure was repeated for each cell. It was made sure that the salt bridge was rinsed with deionised water before placing in a different solution. 3 Instrument parameters Make sure equipment is clean, if not, rinse with tap water then deionised water. Clean the electrodes by dipping them in 6M nitric acid for a few seconds, then rinse with deionised water. When using the Digital Multimeter, make sure a positive sign is shown on screen after the electrodes are connected, if not, switch the leads. Calculations Table 1: Calculation of masses to be used Mass calculated(g) =Molarity x volume x RAM Mass weighed(g) Cu(NO3)2 . 3H2O Zn(NO3)2 . 6H2O Pb(NO3)2 FeSO4 . (NH4)2SO4. 6H2O = 1 x 0.05 x 241.60 = 12.0800 = 1 x 0.05 x 297.46 = 14.8730 = 1 x 0.05 x 331.21 = 16.5605 = 0.5 x 0.05 x 392.14 = 9.8035 = 12.0927 = 14.8679 = 16.5613 = 9.8068 Table 2: Calculation for equal quantities of the copper solution to be used Cu2+(0.1M) Cu2+(0.01M) Cu2+(0.001M) Cu2+(0.05) C1V1=C2V2 0.1 x 0.05 V1= 1 V1= 5 x 10-3 L = 5 ml C1V1=C2V2 0.01 x 0.05 V1= 1 V1= 5 x 10-4 L =0.5 ml C1V1=C2V2 0.001 x 0.05 V1= 1 V1= 5 x 10-5 L =0.05 ml C1V1=C2V2 0.05 x 0.05 V1= 1 V1= 2.5 x 10-3 L =2.5 ml 4 Table 3: Separate calculations Cell No. E0cell = Ecathode - Eanode (Volts) Ecell = E0cell (Volts) 𝟎.𝟎𝟓𝟗𝟐 𝒏 log[Q] emf = |Ecell| 0.0592 1.0 log [1.0] 2 = |0| =0 0.0592 1.0 log [ ] 2 1.0 =|0| =0 0.0592 1.0 log [0.5] 2 =|0.226| =0.226 0.0592 1.0 log [1.0] 2 =|0| =0 0.0592 0.5 log [1.0] 2 =|-0.093| =0.093 0.0592 2 =|0.196| =0.196 1 = 0.34-(-0.76) =1.10 = 1.10 = 0.00 2 = 0.34-(-0.13) =0.47 = 0.47 =0.00 3 = 0.34-(-0.44) =0.78 = 0.78 = 0.226 4 = -0.13-(-0.76) =0.63 = 0.63 = 0.00 5 = -0.44-(-0.76) =0.34 = 0.34 = -0.093 6 = -0.13-(-0.44) =0.31 = 0.31= 0.196 7 = 0.34-(-0.76) =1.10 = 1.10 = -1.070 8 = 0.34-(-0.76) =1.10 = 1.10 = -1.393 9 = 0.34-(-0.76) =1.10 = 1.10 = -2.141 10 = 0.34-(-0.76) =1.10 = 1.10 = -3.211 11 = 0.34-0.34 =0 = 0.78 = -0.75 0.5 log [0.1] 0.0592 0.1 log [1.0] 2 =|-1.070| =1.070 0.0592 0.05 log [ 1.0 ] 2 =-1.393| =1.393 0.0592 0.01 log [ 1.0 ] 2 =|-2.141| =2.141 0.0592 0.001 log [ ] 2 1.0 =|-3.211| =3.211 0.0592 0.1 log [1.0] 2 =|-0.75| =0.75 5 Results Table 4: Measured results Cell No. Anode + Or Or Cathode − Anode + Emf/V Or Or Cathode - Cell Notation 1 Anode − Zn | Zn2+ (1.0M) || Cu2+ (1.0M) | Cu Cathode + 0.876 2 Anode − Pb | Pb2+ (1.0M) || Cu2+ (1.0M) | Cu Cathode + 0.524 3 Anode − Fe | Fe2+ (0.5M) || Cu2+ (1.0M) | Cu Cathode + 0.753 4 Anode − Zn | Zn2+ (1.0M) || Pb2+ (1.0M) | Pb Cathode + 0.4136 5 Anode − Zn | Zn2+ (1.0M) || Fe2+ (0.5M) | Fe Cathode + 0.1411 6 Cathode + Pb | Pb2+ (1.0M) || Fe2+ (0.5M) | Fe Anode − 0.2493 7 Anode − Zn | Zn2+ (1.0M) || Cu2+ (0.1M) | Cu Cathode + 0.4591 8 Anode − Zn | Zn2+ (1.0M) || Cu2+ (0.05M) | Cu Cathode + 0.771 9 Anode − Zn | Zn2+ (1.0M) || Cu2+ (0.01M) | Cu Cathode + 0.7627 10 Anode − Zn | Zn2+ (1.0M) || Cu2+ (0.001M) | Cu Cathode + 0.6154 11 Cathode + Cu | Cu2+ (1.0M) || Cu2+ (0.1M) | Cu Anode − 0.821 Table 5: Calculated results Cell No. Spontaneous Cell Reaction Calculated (Theoretical) Cell Potential/V 1 Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) 2 Pb(s) + Cu2+(aq) → Pb2+(aq) + Cu(s) 3 Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s) 4 Zn(s) + Pb2+(aq) → Zn2+(aq) + Pb(s) 5 Zn(s) + Fe2+(aq) → Zn2+(aq) + Fe(s) 6 Pb(s) + Fe2+(aq) → Pb2+(aq) + Fe(s) = -0.13-(-0.44) =0.31 7 Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) 8 Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) 9 Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) = 0.34-(-0.76) =1.10 = 0.34-(-0.76) =1.10 = 0.34-(-0.76) =1.10 = 0.34-(-0.76) =1.10 = 0.34-(-0.13) =0.47 = 0.34-(-0.44) =0.78 = -0.13-(-0.76) =0.63 = -0.44-(-0.76) =0.34 6 10 Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) 11 Cu(s) + Cu2+(aq) → Cu2+(aq) + Cu(s) = 0.34-(-0.76) =1.10 = 0.34-0.34 =0 Table 6: Required information for the graph below Cell Ecell (measured)/V [Cu2+] No. 0.1 7 = -1.070 0.05 8 = -1.393 = -2.141 0.01 9 0.001 10 = -3.211 log [Cu2+] =log[0.1] = -1 =log[0.05] = -1.301 =log[0.01] = -2 =log[0.001] = -3 Graph 1: A graph comparing Ecell and log[Cu2+] for cells 7,8,9 and 10. A graph comparing Ecell and log[Cu2+] 0 -0,5 -1 -1,5 -1 -1,07 -1,301 -1,393 -2 -2 -2,141 -2,5 -3 -3 -3,211 -3,5 Ecell log[Cu2+] 7 Discussion In this experiment a Digital Multimeter was used to determine the electrode potentials for four metal electrodes (Cu, Zn, Pb and Fe). The electrodes were placed in aqueous solutions, with a salt bridge connected in between. The results obtained were then used as a comparison to the calculated potentials. It is known that if the concentrations of the solutions increase, the cell reaction becomes more spontaneous and the emf increases (Anon. 2006). A positive voltage means the reaction is spontaneous. The concentration trend is seen in the results, for cells 7, 8, 9 and 10. The Nernst equation was used to calculate the cell emf at nonstandard conditions. It helps relate the cell emf (Ecell)to its standard emf(E0cell). In table 3, it shows that the E0 value for copper is 0. This means that the products being made, and reactants being dissociated are happening at the same rate, therefore there is no net potential since the system is already in equilibrium (Tiwari. 2015). Graph 1 was plotted according to the calculations in table 6. It shows that there is a relationship between Ecell and log[Cu2+]. Both line graphs decrease proportionally, in relation to the decrease in concentration. A higher concentration makes the electrode potential more positive and gives it a greater ability to move to equilibrium (Charco. 2007). This is seen at the beginning of the graph, when it starts to curve or level out in a horizontal line. This could have been shown more clearly if more values were taken into consideration. The discrepancies in the results may have been caused by the difference in the calculated mass and the actual mass weighed in table 1, the inaccurate measuring of the solutions using the pipette and when transferring the solutions into different flasks. The difference in the reading time on the Digital Multimeter could have been greater than 30 seconds, and this will cause a great difference since the values on display change constantly. While doing the experiment, it was made sure that all reagents were handled with care and away from our eyes and skin, the salt bridge was washed thoroughly, and all apparatus was clean. Conclusion In conclusion, the experiment was successful and quite accurate because the results measured, and the calculated results are similar. It was also proven that there is a relationship between the Ecell values and concentration. The results have also shown that the concentration effects the emf of a cell and what the emf of a cell is, when it has reached equilibrium. 8 Safety/Precautions Make sure all beakers and flasks are correctly labelled. Dispose all used solutions in an approved manner, as instructed. Make sure the apparatus is cleaned properly after use, to avoid contamination for the next users. Make sure you are dressed appropriately, wearing safety goggles and closed shoes, to avoid chemicals harming your skin. Do not taste of sniff chemicals. Always read the labels on containers, to make sure you are working with the correct reagent. Handle glassware with care. Always wash your hands before leaving the lab. References [1] Anon. 2006. Effect of concentration on cell emf. Available: http://wps.prenhall.com/wps/media/objects/3313/3392587/blb2006.html (Accessed 18 September 2019) [2] Tiwari, C. 2015. What is the emf of the cell when the cell reaction attains equilibrium. Available: https//www.quora.com/what-is-the-EMF-of-the-cell-when-the-cell-reactionattains-equilibrium (Accessed 18 September 2019) [3] Charco. 2007. Effect of concentration on electrode potential. Available: https://www.thestudentroom.co.uk/showthread.php?t=360208 (Accessed 18 September 2019) 9