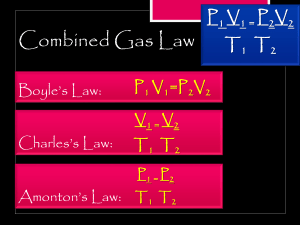
Name: ______________________________________ date: _________________________ Gas laws worksheet science class Ms. Egan Directions: use your textbook to fill in the blanks (pages 69-71), then color the images next to each paragraph. Gas laws Boyle´s Law P1 V1 = P2 V2 When the pressure of a gas at constant ___________________ is increased, the volume of the gas ________________. CHARLES´S LAW V1/T1 = V2/T2 When the temperature of a gas at constant __________________ is increased, its _______________ increases. Questions. Choose the letter of the correct answer. 1. Kevin warmed up a 100-mL sample of air in an expandable container. He displayed the results in the following graph. The results formed a straight line, so he extended the line the full length of the graph. At what temperature did the gas have a volume of 110 mL? A. B. C. D. 2. 19 K 20 K 110 K 300 K Valeria increases the temperature of a gas in an expandable container. If she keeps the pressure constant, what will happen to the volume of the gas? A. B. C. D. The volume will decrease. The volume will increase. The volume will remain the same. The volume will decrease, then increase. Belkis and Aminta performed several experiments to show how changes in temperature affected the volume of an inflated balloon. They made sure the pressure stayed the same. They also showed the effects of pressure on volume if temperature stayed the same. They organized their information in this table. 3. Which experiments provide evidence for Boyle’s law? A. B. C. D. A and B C and D A and C B and D