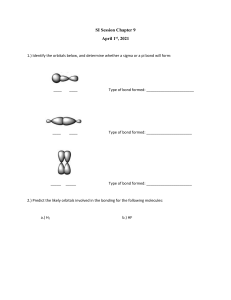
GENERAL CHEMISTRY II CHM 102 LECTURE 5 Ogunjinmi, O.E. Ph.D. HYBRIDIZATION INTRODUCTION Hybridization is an expansion of the valence bond theory. Hybridization is the idea that atomic orbit als fuse or mixed to form newly hybridized orbitals, which in turn, influences molecular geometry an d bonding properties. These orbitals then interact with other atomic orbitals to form chemical bond s. Various molecular geometries can be generated by different hybridizations. The hybridization co ncept accounts for the exception to the octet rule and also explains the formation of double and t riple bonds. Hybridization in Chemistry is defined as the concept of mixing two atomic orbitals to give rise to a n ew type of hybridized orbitals. This intermixing usually results in the formation of hybrid orbitals havin g entirely different energies, shapes, etc. The atomic orbitals of the same energy level mainly take part in hybridization. However, both fully filled and half-filled orbitals can also take part in this proce ss, provided they have equal energy. On the other hand, we can say that the concept of hybridization is an extension of the valence bo nd theory and it helps us to understand the formation of bonds, bond energies and bond lengths. HYBRIDIZATION Number of Orbitals and Types of Hybridization According to VBT theory the metal atom or ion under the influence of ligands can use its (n-1)d, ns, np, or ns, np, nd orbitals for hybridization to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, squa re planar and so on. These hybrid orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding. Coordination Number Type of Hybridisation Distribution of Hybrid Orbitals i n Space 4 sp3 Tetrahedral 4 dsp2 Square planar 5 sp3d Trigonal bipyramidal 6 sp3d2 Octahedral 6 d3sp3 Octahedral HYBRIDIZATION sp3 Hybridization When one ‘s’ orbital and 3 ‘p’ orbitals belonging to the same shell of an atom mix together to form four new equivalent orbital, the type of hybridization is called a tetrahedral hybridization or sp3. The new orbitals formed are called sp3 hybrid orbitals. These are directed towards the four corners of a regular tetrahedron and make an angle of 109°28’ with one another. The angle between the sp3 hybrid orbitals is 109.28 0 Each sp3 hybrid orbital has 25% s character and 75% p character. Example of sp3 hybridization: ethane (C2H6), methane. sp2 Hybridization sp2 hybridization is observed when one s and two p orbitals of the same shell of an atom mix to form 3 equivalent orbitals. The new orbitals formed are called sp2 hybrid orbitals. sp2 hybridization is also called trigonal hybridization. It involves mixing of one ‘s’ orbital and tw o ‘p’ orbital’s of equal energy to give a new hybrid orbital known as sp2. A mixture of s and p orbital formed in trigonal symmetry and is maintained at 1200. All the three hybrid orbitals remain in one plane and make an angle of 120° with one another. Each of the hybrid orbitals formed has a 33.33% ‘s’ character and 66.66% ‘p’ character. The molecules in which the central atom is linked to 3 atoms and is sp2 hybridized have a triangular planar shape. Examples of sp2 Hybridization All the compounds of carbon-containing a carbon-carbon double bond, Ethylene (C2H4) All the compounds of Boron i.e. BF3, BH3 sp Hybridization Sp hybridization is observed when one s and one p orbital in the same main shell of an atom mix to form two new equivalent orbitals. The new orbitals formed are called sp hybridized orbitals. It forms linear molecules with an angle of 180° This type of hybridization involves the mixing of one ‘s’ orbital and one ‘p’ orbital of equal energy to give a new hybrid orbital known as an sp hybridized orbital. Sp hybridization is also called diagonal hybridization. Each sp hybridized orbital has an equal amount of s and p character – 50% s and 50% p character. Examples of sp Hybridization: All compounds of carbon-containing triple bond like C2H2. All ompounds of beryllium like BeF2, BeH2, BeCl2 sp3d Hybridization sp3d hybridization involves the mixing of 1s orbital, 3p orbitals and 1d orbital to form 5 sp3d hybridized orbitals of equal energy. They have trigonal bipyramidal geometry. The mixture of s, p and d orbital forms trigonal bipyramidal symmetry. Three hybrid orbitals lie in the horizontal plane inclined at an angle of 120° to each other known as the equatorial orbitals. The remaining two orbitals lie in the vertical plane at 90 degrees plane of the equatorial orbitals known as axial orbitals. The sp3d hybrid orbitals have 20% 's', 60% 'p' and 20% 'd' characters Example: Hybridization in Phosphorus pentachloride PCl5 sp3d2 hybridization Intermixing of one 's', three 'p' and two 'd' or bitals of almost same energy by giving six id entical and degenerate hybrid orbitals is call ed sp3d2 hybridization. These six sp3d2 orbitals are arranged in oct ahedral symmetry by making 90o angles to each other. This arrangement can be visuali zed as four orbitals arranged in a square pla ne and the remaining two are oriented abov e and below this plane perpendicularly. sp3d3 hybridization In sp3d3 hybridization, one 's', three 'p' and three 'd' orbitals of almost same energy intermix to give seven sp3d3 hybrid orbitals, which are oriented in pentagonal bipyramidal symmetry. Five among the sp3d3 orbitals are arranged in a pentagonal plane by making 72o of angles. The remaining are arranged perpendicularly above and below this pentagonal plane. sp3d2 Hybridization sp3d2 hybridization has 1s, 3p and 2d orbitals, that undergo intermixing to form 6 identica l sp3d2 hybrid orbitals. These 6 orbitals are directed towards the corners of an octahedron. They are inclined at an angle of 90 degrees to one another. Tutorial Questions 1. What is the hybridization in BF3 molecule? (a) sp (b) sp2 (c) sp3 (d) sp3d 2. In the carbonyl group, hybridization of C atom is: (a) sp (b) sp2 (c) sp3 (d) sp3d 3. Give the hybridization states of each of the carbon atoms in t he given molecule. H2C = CH – CN HC ≡ C − C ≡ CH H C = C = C = CH 4. Study the structure below and answer the following questions: (a) How many sigma and pi bonds present in the structure, (b) Identify the chiral carbon. O CH3 Conclusion sp hybridization occurs due to the mixing of one s and one p atomic orbital, sp2 hybridization is the mixing of one s and two p atomic orbitals and sp3 hyb ridization is the mixing of one s and three p atomic orbitals.