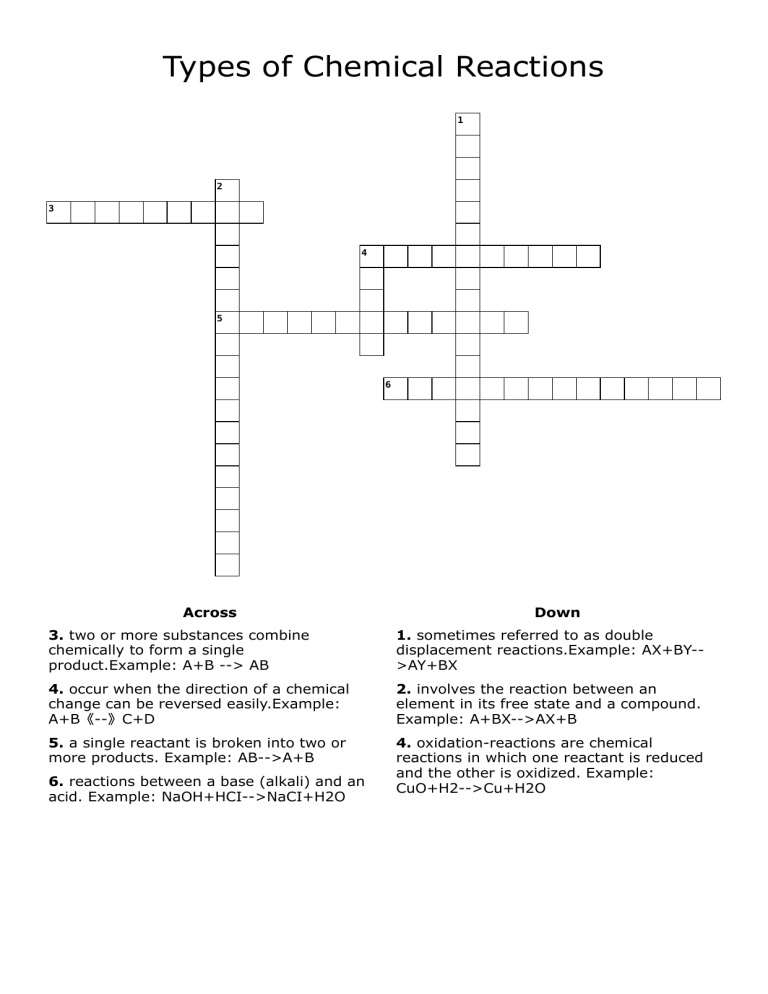
Types of Chemical Reactions 1 2 3 4 5 6 Across Down 3. two or more substances combine chemically to form a single product.Example: A+B --> AB 1. sometimes referred to as double displacement reactions.Example: AX+BY->AY+BX 4. occur when the direction of a chemical change can be reversed easily.Example: A+B《--》C+D 2. involves the reaction between an element in its free state and a compound. Example: A+BX-->AX+B 5. a single reactant is broken into two or more products. Example: AB-->A+B 4. oxidation-reactions are chemical reactions in which one reactant is reduced and the other is oxidized. Example: CuO+H2-->Cu+H2O 6. reactions between a base (alkali) and an acid. Example: NaOH+HCI-->NaCI+H2O