Kinetics equation of different equations:
advertisement
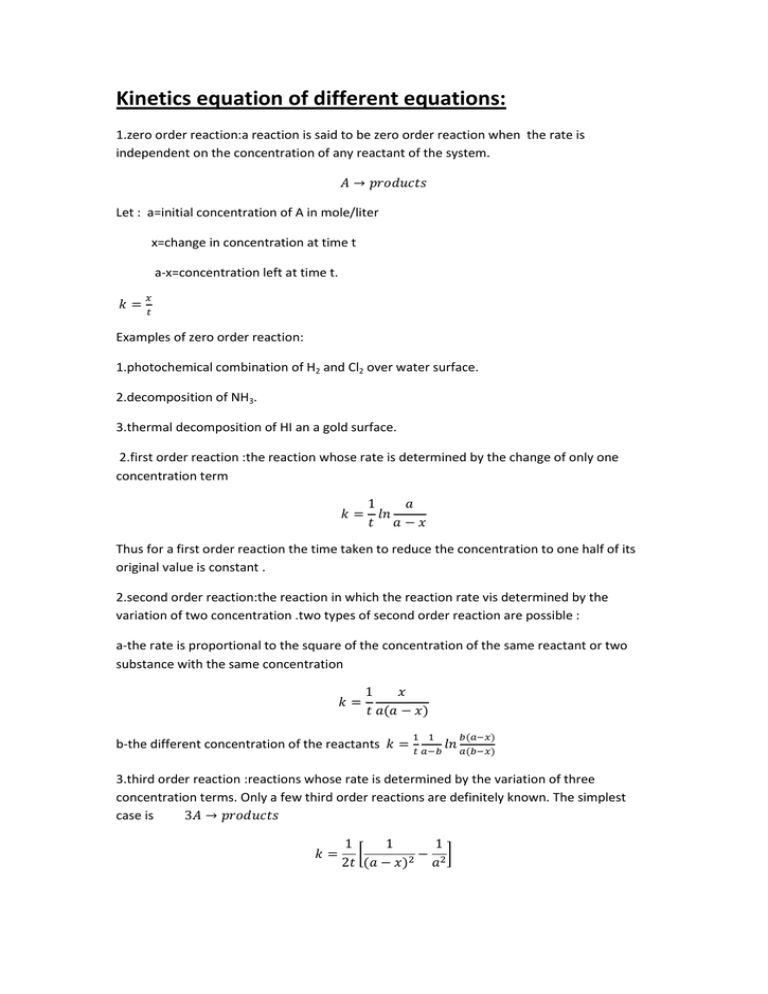
Kinetics equation of different equations: 1.zero order reaction:a reaction is said to be zero order reaction when the rate is independent on the concentration of any reactant of the system. Let : a=initial concentration of A in mole/liter x=change in concentration at time t a-x=concentration left at time t. Examples of zero order reaction: 1.photochemical combination of H2 and Cl2 over water surface. 2.decomposition of NH3. 3.thermal decomposition of HI an a gold surface. 2.first order reaction :the reaction whose rate is determined by the change of only one concentration term Thus for a first order reaction the time taken to reduce the concentration to one half of its original value is constant . 2.second order reaction:the reaction in which the reaction rate vis determined by the variation of two concentration .two types of second order reaction are possible : a-the rate is proportional to the square of the concentration of the same reactant or two substance with the same concentration b-the different concentration of the reactants 3.third order reaction :reactions whose rate is determined by the variation of three concentration terms. Only a few third order reactions are definitely known. The simplest case is [ ]











