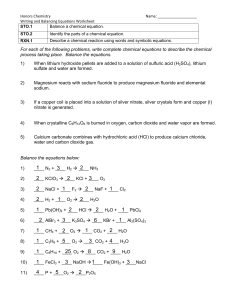
ACTIVITY 3 Chemical Reactions A. DIRECTIONS: Write the balanced equation for each of the following chemical reactions. 1. Potassium chlorate decomposes into potassium chloride and oxygen 2. Silver Nitrate solution, when added to barium chloride solution, forms silver chloride precipitate and aqueous barium nitrate. 3. Nitrogen monoxide reacts with oxygen gas to produce nitrogen dioxide. 4. N2O(g) N2(g) + O2(g) = 5. H2O2(aq) H2O(l) + O2(g) = 6. KHC8H4O4(aq) + NaOH(aq) KNaC8H4O4(aq) + H2O = 7. Zn(s) +HCl(l) ZnCl2(aq) +H2(g) = 8. Na2CO3(aq) + Mg(NO3)2(aq) MgCO3(aq) + NaNO3(aq)= 9. Fe2(s) + O2(g) → Fe2O3(s) = 10. C3H8(g) + O2(g) CO2(g)+ H2O (l) = B. Balance the following chemical reactions, and indicate the type of reaction involved. Type of Reaction 1. NaBr + Ca(OH)2 CaBr2 + NaOH Balanced eq’n: ___________________________ __________________ 2. NH3+ H2SO4 (NH4)2SO4 . BE: __________________________________________ _________________ 3. Al2O3 + H2O � Al(OH)3 BE: ___________________________________________ __________________ 4. Pb + H3PO4 H2 + Pb3(PO4)2 BE: ___________________________________________ __________________ 5. Li3N + NH4NO3 LiNO3 + (NH4)3N BE: ___________________________________________ __________________