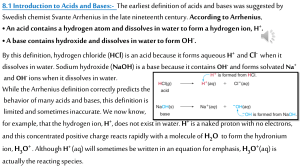
Acid- Base Chemistry Properties of Acid and Base Properties Acid Base Tase Tard/ Sour Bitter Texture Rough Soapy and slippery pH Less than 7 Greater than 7 Turns litmus paper.. Blue Red Red Blue Reactivity – Reacts with metals to produce H2 gas – Reacts with carbonated compounds to produce CO2 – Does not typically react with metals or with carbonated compounds – Does react with oils and fats Conducts electricity in water yes yes Examples vinegar (ethanoic acid) , lemon juice (citric acid) Baking soda (sodium bicarbonate), ammonia water (ammonia hydroxide) 2 Arrhenius Theories of acid and base Arrhenius acids are substances which produce hydrogen ions in solution. HCl(aq) H+(aq) + Cl-(aq) Arrhenius bases are substances which produce hydroxide ions in solution. NaOH(aq) Na+(aq) + OH-(aq) 3 A Brønsted-Lowry Theories of acid and base A Brønsted-Lowry acid is a proton (hydrogen ion) donor. A Brønsted-Lowry base is a proton (hydrogen ion) acceptor. HCl (aq)+NH3 (aq)→NH4+ (aq)+Cl- (aq) A compound that acts as both a Brønsted-Lowry acid and base together is called amphoteric. It can lose or gain a proton, depending on the other reactant. For example, HCO3- acts as an acid in the presence of OH- but as a base in the presence of HF. 4 A strong acid A strong acid is a substance that completely ionizes in aqueous solution to give H3O+(aq) and an anion. HClO4(aq) + H2O(l ) H3O+(aq) + ClO4-(aq) Eg. H2SO4, HCl, HI, HBr, HNO3 A strong base A strong base completely ionizes in aqueous solution to give OHand a cation. NaOH(aq) Na+(aq) + OH-(aq) Eg. hydroxides of Group IA 5 elements and Group IIA elements (except Be). Acid and base are weak which can not completely ionized in solution and exist in reversible reaction with the corresponding ions. CH3COOH (aq) CH3COO- (aq) + H+ (aq) A conjugate acid–base pair consists of two species in an acid–base reaction, one acid and one base, that differ by the loss or gain of a proton. The acid in such a pair is called the conjugate acid of the base, whereas the base is the conjugate base of the acid. A Lewis concept of acid and base Lewis acid is a species that can form a covalent bond by accepting an electron pair from another species. Lewis base is a species that can form a covalent bond by donating an electron pair to another species. Life Science Taking your medicine many medicinal substances are nitrogen bases, substances that organic chemists call amines. Quinine (antimalarial) 8 9 Stronger acids are those lose their protons more easily than other acids. Similarly, the stronger bases are those that hold on to protons more strongly than other bases. The strongest acids have the weakest conjugate bases and the strongest bases have the weakest conjugate acids. 10 Molecular Structure How easily the proton is lost or remove and H-X bond 1. Polarity of the bond. The more polarized the bond, the more easily the proton is removed and the greater the acid strength. 11 2. Strength of the bond The larger the atom X, the weaker the bond and the greater the acid strength. For a series of oxoacids of the same structure, differing only in the atom Y, the acid strength increases with the electronegativity of Y. 12 For a series of oxoacids, (HO)mYOn, the acid strength increases with n, the number of O atoms bonded to Y (excluding O atoms in OH groups). The acid strength of a polyprotic acid and its anions decreases with increasing negative charge 13 Self ionization of water and pH Aqueous solution Hydronium ion (H3O+) or Hydrogen ion (H+) and Hydroxide ion (OH-) Pure water (H2O) non electrolyte but very small conduction self ionization or auto ionization 14