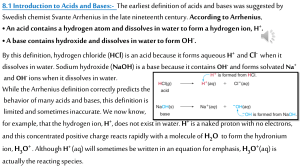
Acids, Bases, and Acid-Base Equilibria Brønsted-Lowry theory • Proposed by J.N. Brønsted in Denmark and T.M. Lowry in Great Britain in 1923. • According to Bronsted-Lowry Theory, ”an acid is a *proton donor and a base is a proton acceptor.” • However, substance cannot simply shed a proton, because proton cannot exist in a solution as a free particle. *Proton is simply an ionized hydrogen atom Conjugate Acid-Base Pair Identify the Brønsted-Lowry acid and base pair and their conjugates in (a) H2S + NH3 (b) OH- + H2PO4(c) NH3 + HCO3(d) H3PO4 + H2O NH4+ + HSH2O + HPO42NH4+ + CO32H2PO4- +H3O+ • Water is an amphiprotic substance – acid and base property, enabling the transfer of proton from one water molecule to another – self-ionization. Self-Ionization of Water – pH Scale Consider the self-ionization of water: 2H2O (l) H3O+ (aq) + OH- (aq) At 25 °C, the experimentally determined equilibrium concentrations in pure water are [H3O+] = [OH-] = 1.0 x 10 -7 M. Determine the [OH-] of HCl if it has 0.00015 M of H3O+? pH/pOH • In 1909, the Danish biochemist Søren Sørenson proposed a convenient convention that is still used today. • pH is refer as “power of hydrogen” • [H+] = 10 –pH • pH = -log [H3O+] Sample Problem 1. Determine the pH of HCl if it has 0.00015 M of H3O+? 2. What is the pH and pOH of a 2.5 x 10 -3 M NaOH solution that has [OH-] = 2.5 X 10-3 ?



![Acid/Base Study Guide Unit 11 Arrhenius pOH = - log[OH ]](http://s2.studylib.net/store/data/017612661_1-9dac3d919856aa6dfb79c8f84ce638fb-300x300.png)