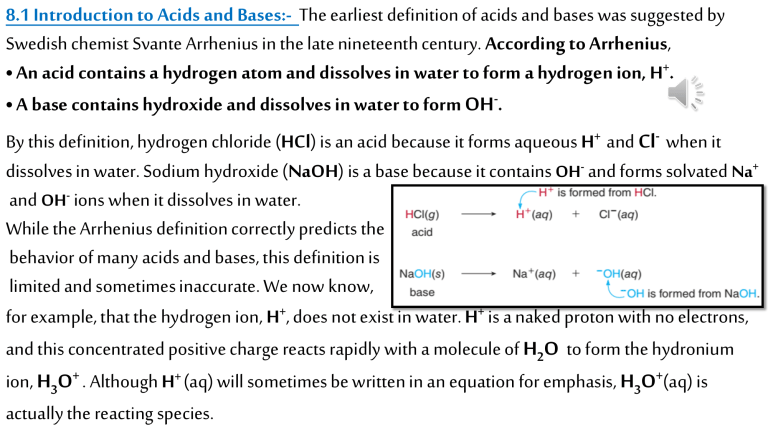
8.1 Introduction to Acids and Bases:- The earliest definition of acids and bases was suggested by Swedish chemist Svante Arrhenius in the late nineteenth century. According to Arrhenius, • An acid contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+. • A base contains hydroxide and dissolves in water to form OH-. By this definition, hydrogen chloride (HCl) is an acid because it forms aqueous H+ and Cl- when it dissolves in water. Sodium hydroxide (NaOH) is a base because it contains OH- and forms solvated Na+ and OH- ions when it dissolves in water. While the Arrhenius definition correctly predicts the behavior of many acids and bases, this definition is limited and sometimes inaccurate. We now know, for example, that the hydrogen ion, H+, does not exist in water. H+ is a naked proton with no electrons, and this concentrated positive charge reacts rapidly with a molecule of H2O to form the hydronium ion, H3O+ . Although H+ (aq) will sometimes be written in an equation for emphasis, H3O+(aq) is actually the reacting species. Moreover, several compounds contain no hydroxide anions, yet they still exhibit the characteristic properties of a base. Examples include the neutral molecule ammonia (NH3) and the salt sodium carbonate (Na2CO3). As a result, a more general definition of acids and bases, proposed by Johannes Brønsted and Thomas Lowry in the early twentieth century, is widely used today. In the Brønsted-Lowry definition, acids and bases are classified according to whether they can donate or accept a proton-a positively charged hydrogen ion, H+ . • A Brønsted-Lowry acid is a proton donor. • A Brønsted-Lowry base is a proton acceptor. • HCl is a Brønsted-Lowry acid because it donates a proton to the solvent water. • H2O is a Brønsted-Lowry base because it accepts a proton from HCl. 8.1A Brønsted-Lowry Acids:A Brønsted-Lowry acid must contain a hydrogen atom. HCl is a Brønsted-Lowry acid because it donates a proton (H+) to water when it dissolves, forming the hydronium ion (H3O+) and chloride (Cl- ). Although hydrogen chloride, HCl, is a covalent molecule and a gas at room temperature, when it dissolves in water it reacts to form two ions, H3O+ and Cl- . An aqueous solution of hydrogen chloride is called hydrochloric acid. Because a Brønsted-Lowry acid contains a hydrogen atom, a general BrønstedLowry acid is often written as HA. A can be a single atom such as Cl or Br. Thus, HCl and HBr are Brønsted-Lowry acids. A can also be a polyatomic ion. Sulfuric acid (H SO ) and nitric acid (HNO )are Brønsted-Lowry acids. Carboxylic acids are a group of Brønsted-Lowry acids that contain the atoms COOH arranged so that the carbon atom is doubly bonded to one O atom and singly bonded to another. Acetic acid, CH3COOH, is a simple carboxylic acid. Although carboxylic acids may contain several hydrogen atoms, the H atom of the OH group is the acidic proton that is donated. Although a Brønsted-Lowry acid must contain a hydrogen atom, it may be a neutral molecule or contain a net positive or negative charge. Thus, H3O+, HCl, and HSO4- are all Brønsted-Lowry acids even though their net charges are +1, 0, and –1, respectively. Vinegar, citrus fruits, and carbonated soft drinks all contain Brønsted-Lowry acids, as shown in Figure 8.1. SAMPLE PROBLEM 8.1:- Which of the following species can be Brønsted-Lowry acids: (a) HF; (b) HSO3- ; (c) Cl2? Analysis:- A Brønsted-Lowry acid must contain a hydrogen atom, but it may be neutral or contain Solution:- a. HF is a Brønsted-Lowry acid since it contains a H. b. HSO3- is a Brønsted-Lowry acid since it contains a H. c. Cl2 is not a Brønsted-Lowry acid because it does not contain a H. PROBLEM 8.1:- Which of the following species can be Brønsted-Lowry acids: (a) HI; (b) SO4-2; (c) H2PO4-; (d) Cl- ? 8.1B Brønsted-Lowry Bases:A Brønsted-Lowry base is a proton acceptor and as such, it must be able to form a bond to a proton. Because a proton has no electrons, a base must contain a lone pair of electrons that can be donated to form a new bond. Thus, ammonia (NH3) is a Brønsted-Lowry base because it contains a nitrogen atom with a lone pair of electrons. When NH3 is dissolved in water, its N atom accepts a proton from H2O, forming an ammonium cation (NH4+) and hydroxide (OH-). A general Brønsted-Lowry base is often written as B: to emphasize that the base must contain a lone pair of electrons to bond to a proton. A base may be neutral or, more commonly, have a net negative charge. Hydroxide (OH-) is the most common Brønsted-Lowry base. The source of hydroxide anions can be a variety of metal salts, including NaOH, KOH, Mg(OH)2 , and Ca(OH)2. Ammonia (NH3) and water (H2O) are both SAMPLE PROBLEM 8.2:Which of the following species can be Brønsted-Lowry bases: (a) LiOH ; (b) Cl-; (c) CH4? Analysis:A Brønsted-Lowry base must contain a lone pair of electrons, but it may be neutral or have a net negative charge. Solution:a. LiOH is a base since it contains hydroxide, OH-, which has three lone pairs on its O atom. b. Cl- is a base since it has four lone pairs. c. CH4 is not a base since it has no lone pairs. PROBLEM 8.2:Which of the following species can be Brønsted-Lowry bases: (a) Al(OH)3 ; (b) Br-; (c) NH4+; (d) CN- ? SAMPLE PROBLEM 8.3:- Classify each reactant as a Brønsted-Lowry acid or base. a. HF(g) + H2O (l) F-(aq) + H3O+ (aq) b. SO4-2(aq) + H2O (l) HSO4- (aq) + OH- (aq) Analysis:- In each equation, the Brønsted-Lowry acid is the species that loses a proton and the Brønsted-Lowry base is the species that gains a proton. Solution:- a. HF is the acid since it loses a proton (H+ ) to form F-, and H2O is the base since it gains a proton to form H3O+. b. H2O is the acid since it loses a proton (H+ ) to form OH- , and SO4-2 is the base since it gains a proton to form HSO4- . 8.2 The Reaction of a Brønsted-Lowry Acid with a Brønsted-Lowry Base:When a Brønsted-Lowry acid reacts with a Brønsted-Lowry base, a proton is transferred from the acid to the base. The Brønsted-Lowry acid donates a proton to the Brønsted-Lowry base, which accepts it. Consider, for example, the reaction of the general acid H A with the general base B:. In an Acid-base reaction, one bond is broken and one bond is formed. The electron pair of the base B: forms a new bond to the proton of the acid, forming BH+. The acid H A loses a proton, leaving the electron pair in the H A bond on A, forming A:• The product formed by loss of a proton from an acid is called its conjugate base. • The product formed by gain of a proton by a base is called its conjugate acid. Thus, the conjugate base of the acid HA is A:-. The conjugate acid of the base B: is BH+. • Two species that differ by the presence of a proton are called a conjugate acid-base pair. Thus, in an acid-base reaction, the acid and the base on the left side of the equation (HA and B:) form two products that are also an acid and a base (BH+ and A:-). When HBr is dissolved in water, for example, the acid HBr loses a proton to form its conjugate base Br- , and the base H2O gains a proton to form H3O+. Thus, HBr and Br- are a conjugate acid-base pair since these two species differ by the presence of a proton (H+). H2O and H3O+ are also a conjugate acid-base pair because these two species differ by the presence of a proton as well. The net charge must be the same on both sides of the equation. In this example, the two reactants are neutral (zero net charge), and the sum of the -1 and +1 charges in the products is also zero. Take particular note of what happens to the charges in each conjugate acid-base pair. When a species gains a proton (H+), it gains a +1 charge. Thus, if a reactant is neutral to begin with, it ends up with a +1 charge. When a species loses a proton (H+), it effectively gains a -1 charge since the product has one fewer proton (+1 charge) than it started with. Thus, if a reactant is neutral to begin with, it ends up The reaction of ammonia (NH3) with HCl is also a Brønsted-Lowry acid-base reaction. In this example, NH3 is the base since it gains a proton to form its conjugate acid, NH4+. HCl is the acid since it donates a proton, forming its conjugate base, Cl- • A Brønsted-Lowry acid-base reaction is a proton transfer reaction since it always results in the transfer of a proton from an acid to a base. SAMPLE PROBLEM 8.4:- Draw the conjugate acid of each base: (a) F-; (b) NO3- . Analysis:- Conjugate acid-base pairs differ by the presence of a proton. To draw a conjugate acid from a base, add a proton, H+ . This adds +1 to the charge of the base to give the charge on the conjugate acid. Solution:- a. F- + H+ gives HF as the conjugate acid. b. NO3- + H+ gives HNO3 (nitric acid) as the conjugate acid. PROBLEM 8.4:- Draw the conjugate acid of each species: (a) H2O; (b) I-; (c) HCO3-. SAMPLE PROBLEM 8.5:- Draw the conjugate base of each acid: (a) H2O; (b) HCO3-. Analysis:- Conjugate acid-base pairs differ by the presence of a proton. To draw a conjugate base from an acid, remove a proton, H+ . This adds -1 to the charge of the acid to give the charge on the conjugate base. Solution:- a. Remove H+ from H2O to form OH-, the conjugate base. b. Remove H+ from HCO3- to form CO3-2, the conjugate base. CO3-2 has a –2 charge since -1 is added to an anion that had a 1 charge to begin with. PROBLEM 8.5:- Draw the conjugate base of each species: (a) H2S; (b) HCN; (c) HSO4-. SAMPLE PROBLEM 8.6:- Label the acid and the base and the conjugate acid and the conjugate base in the following reaction. NH4+(aq) + OH- (aq) NH3(g) + H2O (l) Analysis:- The Brønsted-Lowry acid loses a proton to form its conjugate base. The Brønsted-Lowry base gains a proton to form its conjugate acid. Solution:- NH4+ is the acid since it loses a proton to form NH3, its conjugate base. OH-is the base since it gains a proton to form its conjugate acid, H2O. 8.3 Acid and Base Strength:Although all Brønsted-Lowry acids contain protons, some acids readily donate protons while others do not. Similarly, some Brønsted-Lowry bases accept a proton much more readily than others. How readily proton transfer occurs is determined by the strength of the acid and base. When a covalent acid dissolves in water, proton transfer forms H3O+ and an anion. The splitting apart of a covalent molecule (or an ionic compound) into individual ions is called dissociation. Acids differ in their tendency to donate a proton; that is, acids differ in the extent to which they dissociate in water. • A strong acid readily donates a proton. When a strong acid dissolves in water, essentially 100% of the acid dissociates into ions. • A weak acid less readily donates a proton. When a weak acid dissolves in water, only a small fraction of the acid dissociates into ions. Common strong acids include HI, HBr, HCl, H2SO4 , and HNO3 (Table 8.1). When each acid is dissolved in water, 100% of the acid dissociates, forming H3O+ and the conjugate base, as shown for HCl and H SO . HCl, hydrochloric acid, is secreted by the stomach to digest food (Figure 8.3), and H2SO4, sulfuric acid, is an important industrial starting material in the synthesis of phosphate fertilizers. Acetic acid, CH3COOH, is a weak acid. When acetic acid dissolves in water, only a small fraction of acetic acid molecules donate a proton to water to form H3O+ and the conjugate base, CH3COO-. The major species in solution is the undissociated acid, CH3COOH. Two arrows that are unequal in length are used between the reactants and products to show that both are present in solution. The longer arrow points towards the reactants, since few molecules of acetic acid dissociate. Bases also differ in their ability to accept a proton. • A strong base readily accepts a proton. When a strong base dissolves in water, 100% of the base dissociates into ions. • A weak base less readily accepts a proton. When a weak base dissolves in water, only a small fraction of the base forms ions. The most common strong base is hydroxide, OH-, used as a variety of metal salts, including NaOH and KOH. Solid NaOH dissolves in water to form solvated Na+ cations and OH-anions. In contrast, when NH3, a weak base, dissolves in water, only a small fraction of NH3 molecules react to form NH4+ and OH-. The major species in solution is the undissociated molecule, NH3. An inverse relationship exists between acid and base strength. • A strong acid readily donates a proton, forming a weak conjugate base. • A strong base readily accepts a proton, forming a weak conjugate acid. Why does this inverse relationship exist? Since a strong acid readily donates a proton, it forms a conjugate base that has little ability to accept a proton. Since a strong base readily accepts a proton, it forms a conjugate acid that tightly holds onto its proton, making it a weak acid. Thus, a strong acid like HCl forms a weak conjugate base (Cl- ), and a strong base like OH-forms a weak conjugate acid (H2O). The entries in Table 8.1 are arranged in order of decreasing acid strength. This means that Table 8.1 is also arranged in order of increasing strength of the resulting conjugate bases. Knowing the relative strength of two acids makes it possible to predict the relative strength of their conjugate bases. SAMPLE PROBLEM 8.7:- Using Table 8.1: (a) Is H3PO4 or HF the stronger acid? (b) Draw the conjugate base of each acid and predict which base is stronger. Analysis:- The stronger the acid, the weaker the conjugate base. Solution:- a. H3PO4 is located above HF in Table 8.1, making it the stronger acid. b. To draw each conjugate base, remove a proton (H+). Since HF is the weaker acid, F- is the stronger conjugate base. 8.4 Dissociation of Water:- In Section 8.2 we learned that water can behave as both a Brønsted-Lowry acid and a Brønsted- Lowry base. As a result, two molecules of water can react together in an acid-base reaction. • One molecule of H2O donates a proton (H+), forming its conjugate base OH-. • One molecule of H2O accepts a proton, forming its conjugate acid H3O+. Since water is a very weak acid (Table 8.1), pure water contains an exceedingly low concentration of ions, H3O+ and OH-. Since one H3O+ ion and one OH- ion are formed in each reaction, the concentration of H3O+and OH- are equal in pure water. Experimentally it can be shown that the [H3O+] = [OH-] = 1.0 × 10–7 M at 25 °C. • In pure water, [H3O+] = [OH-] = 1.0 × 10–7 M. Multiplying these concentrations together gives the ion-product constant for water, symbolized by Kw. ubstituting the concentrations for H3O+ and OH-into the expression for Kw gives the following result. S The product, [H3O+][OH-], is a constant, 1.0 × 10–14, for all aqueous solutions at 25 °C. Thus, the value of Kw applies to any aqueous solution, not just pure water. If we know the concentration of one ion, H3O+ or OH-, we can find the concentration of the other by rearranging the expression for Kw. • Thus, if the concentration of H3O+ in a cup of coffee is 1.0 × 10–5 M, we can use this value to calculate [OH-]. In a cup of coffee, therefore, the concentration of H3O+ ions is greater than the concentration of OHions, but the product of these concentrations, 1.0 × 10–14, is a constant, Kw. Pure water and any solution that has an equal concentration of H3O+ and OH-ions (1.0 × 10–7) is said to be neutral. Other solutions are classified as acidic or basic, depending on which ion is present in a higher concentration. • In an acidic solution, [H3O+] > [OH-]; thus, [H3O+] > 10–7 M. • In a basic solution, [OH-] > [H3O+]; thus, [OH-] > 10–7 M. In an acidic solution, the concentration of the acid H3O+is greater than the concentration of the base OH-. In a basic solution, the concentration of the base OH-is greater than the concentration of the acid H3O+. Table 8.2 summarizes information about neutral, acidic, and basic solutions. SAMPLE PROBLEM 8.8:- If [H3O+] in blood is 4.0 × 10–8 M, what is the value of [OH-]? Is blood acidic, basic, or neutral? Analysis:- Use the equation [OH-] = Kw/[H3O+] to calculate the hydroxide ion concentration. Solution:- Substitute the given value of [H3O+] in the equation to find [OH-]. Since [OH-] > [H3O+], blood is a basic solution. PROBLEM 8.12- Calculate the value of [OH-] from the given [H3O+] in each solution and label the solution as acidic or basic: (a) [H3O+] = 10–3 M; (b) [H3O+] = 10–11 M; (c) [H3O+] = 2.8 × 10–10 M; (d) [H3O+] = 5.6 × 10–4 M. PROBLEM 8.13:- Calculate the value of [H3O+] from the given [OH-] in each solution and label the solution as acidic or basic: (a) [OH-] = 10–6 M; (b) [OH-] = 10–9 M; (c) [OH-] = 5.2 × 10–11 M; (d) [OH-] = 7.3 × 10–4 M. Since a strong acid like HCl is completely dissociated in aqueous solution, the concentration of the acid tells us the concentration of hydronium ions present. Thus, a 0.1 M HCl solution completely dissociates, so the concentration of H3O+ is 0.1 M. This value can then be used to calculate the hydroxide ion concentration. Similarly, a strong base like NaOH completely dissociates, so the concentration of the base gives the concentration of hydroxide ions present. Thus, the concentration of OH-in a 0.1 M NaOH solution is 0.1 M. SAMPLE PROBLEM 8.9:- Calculate the value of [H3O+] and [OH-] in a 0.01 M NaOH solution. Analysis:- Since NaOH is a strong base that completely dissociates to form Na+ and OH-, the concentration of NaOH gives the concentration of OH-ions. The [OH-] can then be used to calculate [H3O+] from the expression for Kw. Solution:The value of [OH-] in a 0.01 M NaOH solution is 0.01 M = 1 × 10–2 M.