HW9.docx
advertisement

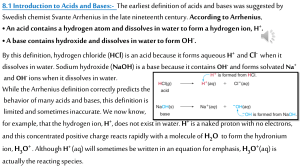
Name ________________ CHEM 1004 Homework #9 Fall 2010 (Buckley) This homework is due on Tuesday, November 2, at class time. 1. (10 points) For each of the following species: a. Indicate whether it could act as a Brønsted-Lowry acid, a Brønsted-Lowry base, or either. b. If it is a Brønsted-Lowry acid, write the formula for the conjugate base; if it is a Brønsted-Lowry base, write the formula for the conjugate acid; if it could act as either a Brønsted-Lowry acid or a Brønsted-Lowry base, write the formula for its conjugate base. Don’t forget to include the correct charges on the formulas. Species Acid, Base, or Both? Formula for Conjugate HNO3 H2CO3 NH3 C6H5COOH (only the last H can come off) CH3COOH (only the last H can come off) 2. (4 points) Complete and balance each of the following reactions. SO3 + H2O → MgO + H2O → 3. (6 points) Write the chemical equation representing the reaction between the following acid/base pairs. a. HCl and Ba(OH)2 b. H2SO3 and NaOH c. H2CO3 and Sr(OH)2 4. (2 point) The pH of a solution that has a hydrogen ion concentration of 4 × 10-6 M is most near: a. 1.5 b. 4.5 c. 5.5 d. 6.5 e. 10.5 5. (2 points) The solution in problem 4 is _______________ (acidic, basic, or neutral) 6. (2 points) Approximately what is the hydrogen concentration in a solution with a pH of 10.8? a. 2 × 10-4 M b. 2 × 10-6 M c. 2 × 10-8 M d. 2 × 10-10 M e. 2 × 10-11 M 7. (4 points) It turns out that the conjugate bases of strong acids are weak bases and the conjugate acids of strong bases are weak bases. Determine whether each of the following would be expected to be a strong or weak base. Explain your reasoning. a. Br - b. F-