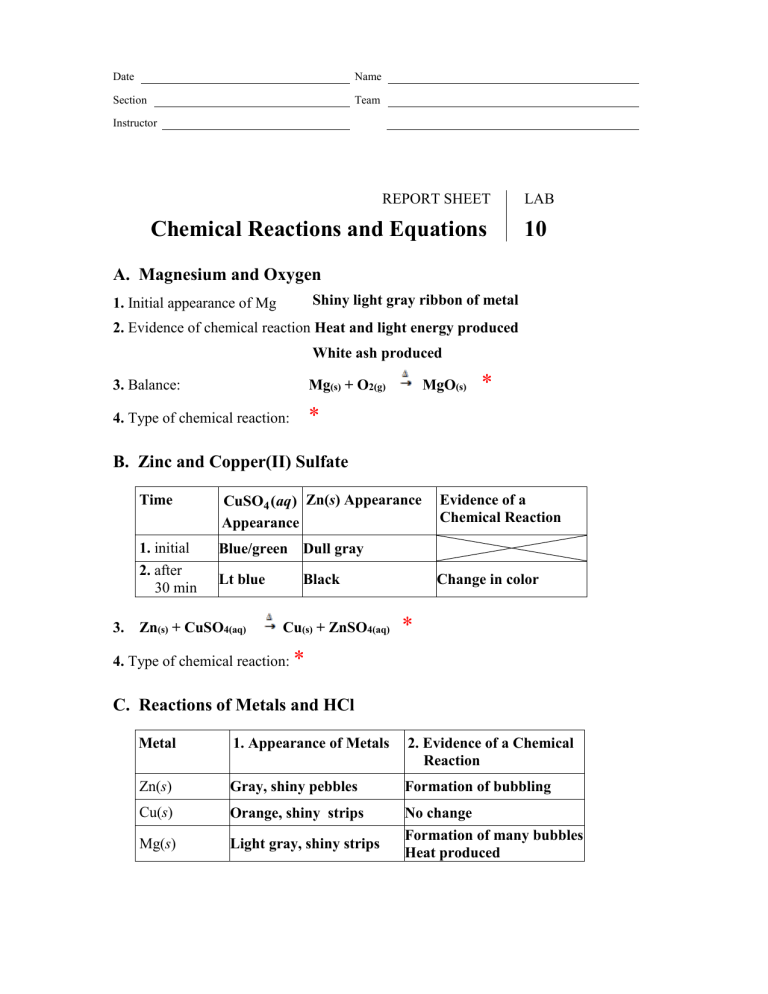
Date Name Section Team Instructor REPORT SHEET Chemical Reactions and Equations LAB 10 A. Magnesium and Oxygen Shiny light gray ribbon of metal 1. Initial appearance of Mg 2. Evidence of chemical reaction Heat and light energy produced White ash produced 3. Balance: Mg(s) + O2(g) 4. Type of chemical reaction: * MgO(s) * B. Zinc and Copper(II) Sulfate Time CuSO4 (aq ) Zn(s) Appearance Appearance 1. initial 2. after 30 min Blue/green Dull gray Lt blue 3. Zn(s) + CuSO4(aq) Black Cu(s) + ZnSO4(aq) Evidence of a Chemical Reaction Change in color * 4. Type of chemical reaction: * C. Reactions of Metals and HCl Metal 1. Appearance of Metals 2. Evidence of a Chemical Reaction Zn(s) Gray, shiny pebbles Formation of bubbling Cu(s) Orange, shiny strips Mg(s) Light gray, shiny strips No change Formation of many bubbles Heat produced 3. Zn(s) + HCl(aq) ZnCl2(aq) + H2(g) Cu(s) + HCl(aq) * NR Mg(s) + HCl(aq) MgCl2(aq) + H2(g) * 4. Type of chemical reaction: Zn: * Cu: No apparent reaction Mg: * D. Reactions of Ionic Compounds D1 Reaction of CaCl 2 and Na3PO4 Reactants 1. Appearance of Solutions CaCl2 (aq) Clear and colorless liquid Na 3PO4 (aq) Clear and colorless liquid 3. CaCl2(aq) + Na3PO4(aq) 2. Evidence of a Chemical Reaction Formation of a white solid Ca3(PO4)2(s) + NaCl(aq) * 4. Type of reaction:: * D2 Reaction of FeCl 3 and KSCN Reactants 1. Appearance of Solutions 2. Evidence of a Chemical Reaction FeCl3 (aq) Orange liquid KSCN( aq) Clear, colorless liquid Change in color to blood red 3. FeCl3(aq) + KSCN(aq) Fe(SCN)3(aq) + KCl(aq) * 4. Type of reaction: * E. Sodium Carbonate and HCl Reactants 1. Appearance of Reactants HCl(aq ) Clear, colorless liquid Na 2CO3 ( s) White, crystalline solid 2. Evidence of a Chemical Reaction Formation of many bubbles 3. Observation of burning match or splint: It immediately went out. What caused the change in the burning match or splint? Lack of oxygen from the CO2 gas formation, displacing air. 4. Na2CO3(s) + HCl(aq) CO2(g) + H2O(l) + NaCl(aq) * 5. Type of reaction: * F. Hydrogen Peroxide Reactants 1. Appearance of Reactants 2. Evidence of a Chemical Reaction H 2O2 (aq) Clear, colorless liquid Formation of bubbles * 4. Type of chemical reaction: * 3. H2O(l) H2O(l) + O2(g) Questions and Problems Q1 What evidence of a chemical reaction might you see in the following cases? Refer to Table 10.1. a. dropping an Alka-Seltzer tablet into a glass of water * b. bleaching a stain * c. burning a match * d. rusting of an iron nail * Q2 Balance the following equations: a. Mg(s) + HCl(aq) H2(g) + MgCl2(aq) Al2O3(s) b. Al(s) + O2(g) * * c. Fe2O3(s) + H2O(l) Fe(OH)3(s) * Ca(NO3)2(aq) + H2O(l) d. Ca(OH)2(aq) + HNO3(aq) * Q3 Write a balanced equation for each of the following reactions. Write the correct formulas of the reactants and products and the states of each. a. Liquid pentane (C5H12 ) and oxygen (O 2 ) gas react to form carbon dioxide and water. C5H12(l) + O2 (g) CO2(g) + H2O(l) * b. Sodium and water react to form sodium hydroxide and hydrogen gas (H 2 ). Na(s) + H2O(l) NaOH(aq) + H2(g) * c. Iron and oxygen (O 2 ) gas react to form iron(III) oxide. Fe(s) + O2(g) Fe2O3(s) * Q4 Classify each reaction as combination, decomposition, single replacement, double replacement, or combustion. a. Ni(s) F2 (g ) NiF2 (s) * b. Fe2O3 (s) 3C(s) 2Fe(s) 3CO(g ) * c. CaCO3 (s) CaO(s) CO2 (g ) * d. H2SO4 (aq) 2KOH(aq) K 2SO4 (aq) 2H 2O(l ) * e. C2H4 (g ) 3O2 (g ) 2CO2 (g ) 2H 2O(g ) * Q5 Complete and balance each of the following chemical reactions by writing the correct formulas of the product(s) that forms: Type of Reaction a. 2 Zn(s) + 1 CuBr2(aq) 2 ZnBr(aq) + 1 Cu(s) 2 HCl(aq) b. 1 H2(g) + 1 Cl2(g) c. 1 MgCO3(s) 1 MgO(s) + 1 CO2(g) 1 KNO3(aq) + 1 AgCl(s) d. 1 KCl(aq) + 1 AgNO3(aq) * * * *