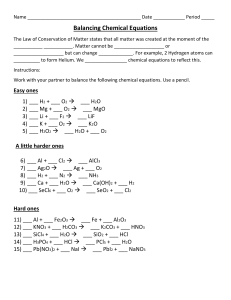
Basic oxides Continue equations 1-6 by following the respective general equations. Equations 1-3 can be continued using reaction 1, while equations 4-6 can be continued by using reaction 2. Reaction 1 Basic oxide+ water→ metal hydroxide 1. K2O+H2O → 2. BaO+H2O → 3. Rb2O+H2O → Reaction 2 Basic oxide+ acid→ salt + water 4. CaO+H2SO4→ 5. K2O+HNO3 → 6. Na2O+H2SO4→ Note : the salt is formed when the metal replaces the hydrogen from the acid.