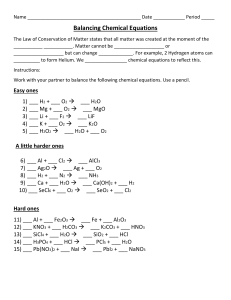
Part 1: Balancing and Writing Equations For each of the following problems, write complete chemical equations (formulas) to describe the chemical process taking place. Balance the equations. Use → for your arrows 1. When lithium hydroxide pellets are added to a solution of sulfuric acid (H2SO4), lithium sulfate and water are formed. 2. Magnesium metal reacts with sodium fluoride to produce magnesium fluoride and elemental sodium. 3. If a copper coil is placed into a solution of silver nitrate, silver crystals form and copper (I) nitrate is generated. 4. Balance the equations below: 1) ____ N2 + ____ H2 →____ NH3 2) ____ KClO3 →____ KCl + ____ O2 3) ____ NaCl + ____ F2 →____ NaF + ____ Cl2 4) ____ H2 + ____ O2 → ____ H2O 5) ____ Pb(OH)2 + ____ HCl →____ H2O + ____ PbCl2 6) ____ AlBr3 + ____ K2SO4 →____ KBr + ____ Al2(SO4)3 7) ____ CH4 + ____ O2 →____ CO2 + ____ H2O 8) ____ C3H8 + ____ O2 → ____ CO2 + ____ H2O 9) ____ C8H18 + ____ O2 → ____ CO2 + ____ H2O 10) ____ FeCl3 + ____ NaOH →____ Fe(OH)3 + ____NaCl 11) ____ P + ____O2 → ____P2O5 Part 2: Molar Ratio Practice Problems Following each equation are two requests for molar ratios from the equation. 1) N2 + 3 H2 → 2 NH3 1:3 N2 to H2: 2:3 NH3 to H2: 2) 2 SO2 + O2 → 2 SO3 O2 to SO3: O2 to SO2: 3) PCl3 + Cl2 → PCl5 PCl3 to Cl2: PCl3 to PCl5: 4) 4 NH3 + 3 O2 → 2 N2 + 6 H2O NH3 to N2: H2O to O2: 5) Fe2O3 + 3 CO → 2 Fe + 3 CO2 CO to CO2: Fe to CO: Mole to Mole Practice Problems Here's the equation to use for all three problems: 2 H2 + O2 → 2 H2O 1) How many moles of H2O are produced when 5.00 moles of oxygen are used? Remember: Starting with five moles of oxygen and based on the balanced equation, for every 1 mole of oxygen used, two moles of water are produced. _______ moles O2 x 2 mole H2O = 1 mole O2 moles H2O water 2) If 3.00 moles of H2O are produced, how many moles of oxygen must be consumed? 3) If 2.50 moles of H2O are produced, how many moles of hydrogen gas must be used? 4) Suppose 0.316 moles of H2 were used? How many moles of water would be produced?