Fluid Dynamics Assignment: Fluidized Beds & Pressure Drop
advertisement

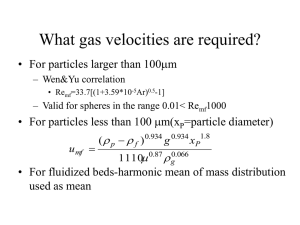
Chemical Engineering Fluid Dynamics Assignment 01 Submitted to: Sir Usman Asghar Submitted by: Muhammad Daniyal Roll No. UW-19-CHE-BSc-024 (Department of Chemical Engineering) Wah Engineering College University of Wah 1. Explain the phenomena of flow of fluid through beds. A fluidized bed is a physical phenomenon occurring when a quantity of a solid particulate substance (usually present in a holding vessel) is placed under appropriate conditions to cause a solid/fluid mixture to behave as a fluid. This is usually achieved by the introduction of pressurized fluid through the particulate medium. This results in the medium then having many properties and characteristics of normal fluids, such as the ability to free-flow under gravity, or to be pumped using fluid type technologies. The resulting phenomenon is called fluidization. Fluidized beds are used for several purposes, such as fluidized bed reactors (types of chemical reactors), solids separation, fluid catalytic cracking, fluidized bed combustion, heat or mass transfer or interface modification, such as applying a coating onto solid items. This technique is also becoming more common in aquaculture for the production of shellfish in integrated multi-trophic aquaculture systems. 2. Equations used to find the pressure drop through bed of solids. Ergun Equation is used to Find pressure Drop through bed of solids: Where µ Is the Dynamic Viscosity ₯ Diameter of particles of beds L Length of bed ρ Density of fluid є is the void fraction (porosity) of the bed. vs is the superficial velocity (i.e. the velocity that the fluid would have through the empty tube at the same volumetric flow rate) 3. Different regimes of particulate and aggregative fluidization. Fluidization regimes Geldart2 classified the fluidization of particles into four groups which are used extensively in the industry. The following are the major groups: Group A Small particle size or density less than 1.4 g/cm3 . Easily fluidized with smooth fluidization at low gas velocities and controlled bubbling with small bubbles at higher gas velocities. When fluidized by air at ambient conditions, result in a region of non-bubbling fluidization beginning at Umf , followed by bubbling fluidization as fluidizing velocity increases. Gas bubbles rise faster than the rest of the gas. Major example is the FCC catalyst. Group B are sand like powders which result in vigorous bubbling fluidization under these conditions. Bubbles form as soon as the gas velocity exceeds the minimum fluidization velocity. Majority of gas-solid reactions occur in this regime based on particle size of raw materials. Group C cohesive, or very fine powders. Normal fluidization is difficult for these solids because of interparticle forces that are greater than those resulting from the action of the gas on the particles. In small diameter beds these particles form a plug of solids that rises upward. powders - very fine, cohesive powders which are incapable of fluidization in the strict sense. Examples: Face powder, flour, and starch. Group D spoutable, large and/or dense particles as shown in Figure 2. Examples include drying grains, peas, roasting coffee beans, gasifying coals and roasting of metal ores.