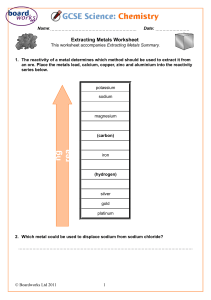
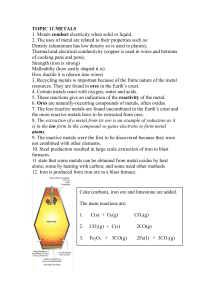
Chapter 15 Extraction and Uses of Metals ➢ Ores are naturally occurring rocks that contain metals or metal compounds in sufficient amounts to make it worthwhile extracting them. ➢ A very few unreactive metals such as gold exist naturally as uncombined element. These metals are found native. Silver and copper are sometimes found native, ➢ The method used to extract a given metal from its ore depends on: • The reactivity of the metal, and • How stable the ore is. How metals can be extracted? 1. Ores are either oxides/ compounds that can be easily converted into oxides by heating them in air (roasting). 2. We can now remove the oxygen from the metal oxide by reduction (Revise the concept of oxidation and reduction before progressing!!) ➢ Metals below carbon are extracted by heating it with carbon. For example Haematite is a reddish black mineral consisting of ferric oxide (Fe2O3). ➢ It can be extracted by heating the metal with carbon. Fe2O3 (s) + 3C (s) → 2Fe(s) + 3CO (g) ➢ Since carbon is higher in the reactivity series than iron, it will take oxygen from iron and gets oxidized. Iron is being reduced in the reaction. ➢ Carbon is acting as the reducing agent. ➢ Extraction of iron is carried out in a blast furnace. The process is complicated but we do not need to know this in detail. ➢ Diagram of blast finance ( Page 162 Student’s book) . 1