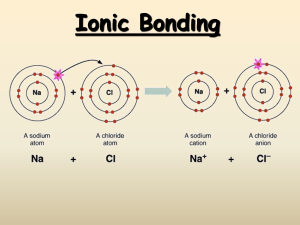
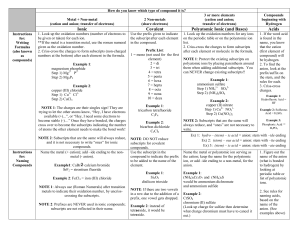
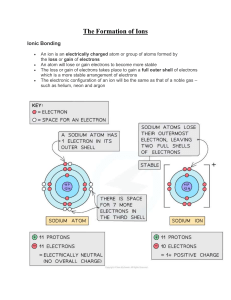
Ions Worksheet Directions: Using your periodic table, identify how many valence electrons each element contains. After that determine whether the atom will want to lose or gain electrons in order to be stable when bonding with another elements. Identify the new ion and whether it is a cation or anion. Element # Valence Electrons # Electrons to gain # Electrons to lose Ion Formed/ name Li 1 N 5 None 3 1 None Li +1 / cation –3 N /anion O Ca Br S Cl K Mg Be Questions 1. What types of elements make up ionic compounds? 2. How do ionic compounds form? 3. What state of matter are most ionic compounds at room temperature? 4. How is the melting point and boiling point of ionic compound? (is it high?, low? Or varies between elements? 5. Can ionic compounds conduct electricity? If so, what needs to happen to them? 6. If Li loses an electron to another atom, why is it written Li + 1 (with a +1)? 7. If N gains 3 electrons from other atoms, why is it written N-3 (with a -3)?