Ionic Bonds Explained: Cations, Anions, and Electrostatic Attraction
advertisement

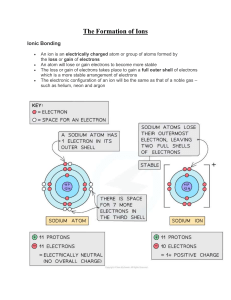
Ionic Bonds First Video: Ionic bonds give rise to compounds known as ionic or electrovalent compounds, best exemplified by compounds formed between non-metals and alkaline and alkaline earth metals. In such an ionic crystalline solid, the electrostatic attraction between the opposite charges and the repulsive force between the charges causes the ions to be oriented so that the positive ions are surrounded by negative ions or vice versa. Second Video: Ionic bonding is the complete movement of valence electrons between atoms. This is a type of chemical bond that creates two oppositely charged ions. In an ionic bond, a metal loses an electron and becomes a positively charged cation, and a non-metal acquires those electrons and becomes a negatively charged anion. Third Video: Ionic compounds are ion compounds. These ions are atoms that gain or lose electrons and carry a net positive or negative charge. Metals tend to lose electrons, so they have a net positive charge and become cations. Nonmetals tend to accept electrons and produce a net negative charge for anions.