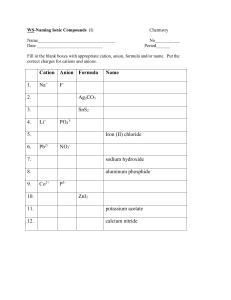
How do you know which type of compound it is? Metal + Non-metal (cation and anion; transfer of electrons) Instructions for: Writing Formulas (also known as compounds) 3 or more elements (cation and anion; transfer of electrons) Compounds beginning with Hydrogen Ionic Covalent Polyatomic Ionic (and Bases) Acids 1. Look up the oxidation numbers (number of electrons to be given or taken) for each ion. **If the metal is a transition metal, use the roman numeral given as the oxidation number. 2. Criss-cross the charges to form subscripts (non-charged numbers at the bottom) after each element in the formula. Use the prefix given to indicate the subscript after each element in the compound. 1. Look up the oxidation numbers for any ions on the periodic table or on the polyatomic ion list. 2. Criss-cross the charges to form subscripts after each element or molecule in the formula. 1. If the word acid is found in the name, you know that the cation (first element of compound) will be hydrogen. 2. To find the anion, look at the prefix/suffix on the stem, and the rules for each. 3. Criss-cross charges. Example 1: magnesium phosphide Step 1) Mg2+ P3Step 2) Mg3P2 Example 2: copper (III) chloride Step 1) Cu3+ Cl1Step 2) CuCl3 Instructions for: Naming Compounds 2 Non-metals (share electrons) Prefix List: 1 = mono (not used for the first element) 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca NOTE 1: The charges are their singles sign! They are trying to let the other atoms know, “Hey, I have electrons available (+)...”, or “Hey, I need some electrons to become stable (-)…” Once they have bonded, the charges cross over to become the subscripts indicating the number of atoms the other element needs to make the bond work! Example 1: tricarbon tetrafluoride C3F4 NOTE 2: Subscripts that are the same will always reduce, and it is not necessary to write “ones” for ionic compounds. Name the metal (+ cation); add –ide ending to the nonmetal (- anion). NOTE: DO NOT reduce subscripts for covalent compounds. Use the subscript in the compound to indicate the prefix to be added to the name of the element. Example1: CaBr 2= calcium bromide SrF2 = strontium fluoride Example 2: FeCl3 = iron (III) chloride NOTE 1: Always use (Roman Numerals) after transition metals to indicate their oxidation number, by uncrisscrossing the subscripts. NOTE 2: Prefixes are NEVER used in ionic compounds; subscripts are not reflected in their name. Example 2: bicarbon dichloride C2Cl2 Example 1: Si2O3 disilicon trioxide NOTE: If there are two vowels in a row due to the addition of a prefix, one vowel gets dropped. Example 2: instead of tetraoxide, it would be tetroxide. NOTE 1: Protect the existing subscripts on polyatomic ions by placing parenthesis around them when adding additional subscripts. You can NEVER change existing subscripts!! Example 1: ammonium sulfate Step 1) NH41+ SO42Step 2) (NH4)2SO4 Example 2: copper (II) nitrate Step 1) Cu2+ NO31Step 2) Cu(NO3)2 NOTE 2: Subscripts that are the same will always reduce, and “ones” are not necessary to write. Example 1: Hydrofluoric Acid = HF Example 2: Sulfurous Acid = H2SO3 Example 3: Phosphoric Acid H3PO4 = Ex) 1: hydro – (stem) – ic acid = anion: stem with –ide ending Ex) 2: (stem) – ous acid = anion: stem with –ite ending Ex) 3: (stem) – ic acid = anion: stem with –ate ending Name the metal or polyatomic ion serving as the cation; keep the name for the polyatomic ion, or add –ide ending to a non-metal, for the anion. Example 1: (NH4)2Cr2O7 and (NH4)2S would be ammonium dichromate and ammonium sulfide Example 2: CrSO4 chromium (II) sulfate (Look up charge for sulfate then determine what charge chromium must have to cancel it out.) 1. Figure out the name of the anion (what is bonded to hydrogen) by looking at periodic table or list of polyatomic ions. 2. See rules for naming acids, based on the name of the anion. (See examples above)