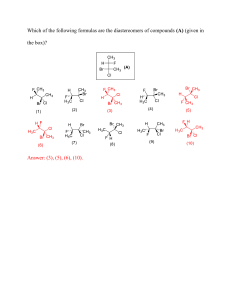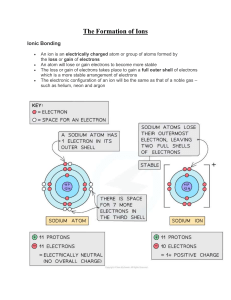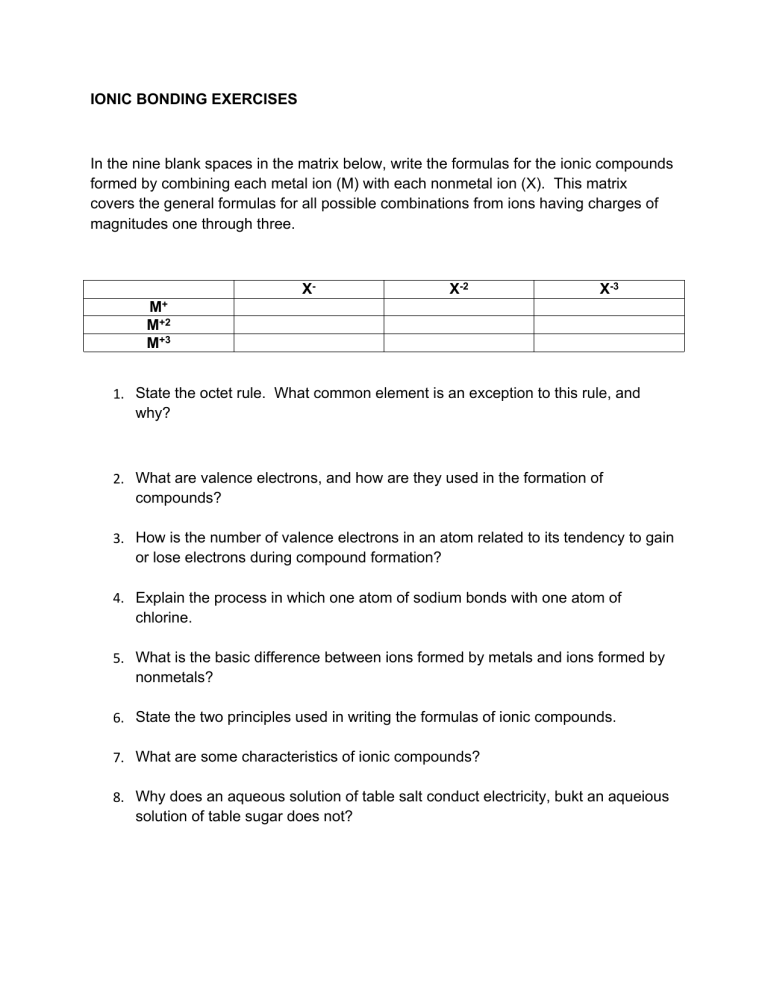
IONIC BONDING EXERCISES In the nine blank spaces in the matrix below, write the formulas for the ionic compounds formed by combining each metal ion (M) with each nonmetal ion (X). This matrix covers the general formulas for all possible combinations from ions having charges of magnitudes one through three. X- X-2 X-3 M+ M+2 M+3 1. State the octet rule. What common element is an exception to this rule, and why? 2. What are valence electrons, and how are they used in the formation of compounds? 3. How is the number of valence electrons in an atom related to its tendency to gain or lose electrons during compound formation? 4. Explain the process in which one atom of sodium bonds with one atom of chlorine. 5. What is the basic difference between ions formed by metals and ions formed by nonmetals? 6. State the two principles used in writing the formulas of ionic compounds. 7. What are some characteristics of ionic compounds? 8. Why does an aqueous solution of table salt conduct electricity, bukt an aqueious solution of table sugar does not? 9. Referring only to a periodic table, give the ionic charge expected for each of these representative elements: (a) S, (b) K, (c) Br, (d) N, (e) Mg, (f) Ne, (g) C, and (h) Al. 10. Calculate the formula mass of these compounds: a. b. c. d. Lithium chloride, LiCl Silver carbonate, Ag2CO3 Zinc nitrate, Zn(NO3)2 Methane, CH4 11. Predict the formula for each ionic compound: a. Cesium iodide b. Barium fluoride c. Aluminum chloride d. Lithium sulfide e. Beryllium oxide f. Sodium nitride