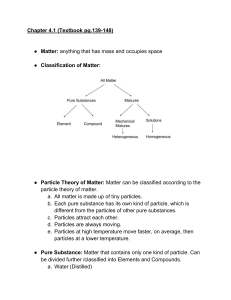
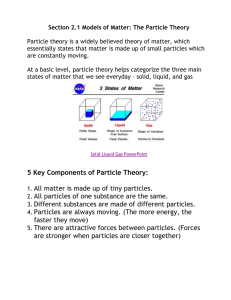
MATTER • Matter is defined as anything that has mass and takes up space Particle Theory #1 All matter is made of tiny particles that have empty spaces between them. Particle Theory #2 Different substances are made of different kinds of particles Particle Theory #3 Particles are in constant random motion. Particle Theory #4 The particles of a substance move faster as the temperature increases. Particle Theory #5 Particles attract each other – the closer together, the stronger the attraction. States of Matter PROPERTIES SOLID STATES OF MATTER LIQUID GAS Weaker than solids Weakest (almost none) Particle Arrangement Forces between particles Motion of particles Shape Can be Compressed? Expansion (volume) when heated Strongest Particles can vibrate, but do not move from their position Fixed shape No Expands slightly Particles move Particles are able about very fast to move around in all directions No fixed shape; takes the shape of container No (negligible) Expands more than solids but less than gases No fixed shape; takes the shape of container Yes Expands more than solids and liquids Changes of State Evaporation Condensation Sublimation Sublimation Melting Freezing Classification of Matter Classification of Matter Matter can be classified into two main categories-pure substances and mixtures - based on what types of particles make up the matter. When a substance consists of only one type of particle, it is a pure substance. Examples of pure substances include distilled water, carbon dioxide, and pure iron. When a substance consists of more than one type of particle, it is a mixture. Examples of mixtures include granola bars, orange juice, tea, and soil. Classification of Mixtures Mixtures can be further classified into mechanical mixtures and solutions We can visually distinguish the different substances in a mechanical mixture. Examples of mechanical mixtures include cereal and Caesar salad. The different substances in a solution cannot be visually distinguished. A solution looks like a pure substance, but it is not. Examples of solutions include apple juice and non-distilled water. An alloy is a type of solution in which two or more metals are mixed together. Examples of alloys include bronze and stainless steel. Classify the following as a pure substance, mechanical mixture or solution: 1. Spicy Salad dressing ____________________ Mechanical mixture Pure substance 2. Gold bars at Fort Knox ____________________ Solution 3. A glass of KoolAid ____________________ Pure substance 4. Copper wire ____________________ 5. A quarter ____________________ Pure substance Solution 6. A cold drink of iced tea ____________________ Solution 7. Air ____________________ Mechanical mixture 8. Cake mix ____________________ Pure substance 9. Distilled water ____________________ Mechanical mixture 10.A compost pile in the garden ____________________