Chemistry Reaction Rates Test - High School
advertisement

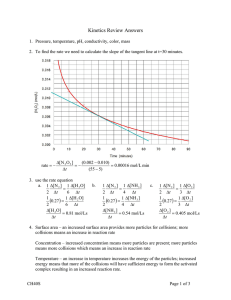
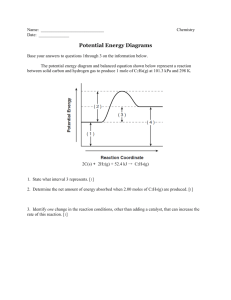
CHAPTER 6 I ____ ____ ____ ____ ____ Multiple Choice dentify the letter of the choice that best completes the statement or answers the question. 1. A rate of reaction is usually obtained by measuring a. the rate at which products are consumed b. the rate at which reactants are produced c. the rate at which reactants are consumed d. the temperature of the solution e. the mass and volume of the products 2. The following property can be measured to determine the rate of the reaction a. change in mass b. change in colour c. change in volume d. change in pressure e. all of the above depending on the reaction 3. In the reaction, , if the concentration changes from 0.45 mol/L to 1.0 in 2 minutes, what is the overall rate of production of nitrogen dioxide in the system? a. 3.64 mol/(L·min) d. 12.6 mol/(L·min) b. 0.275 mol/(L·min) e. 0.333 mol/(L·min) c. 0.137 mol/(L·min) 4. What is the overall rate of change in the combustion reaction of propane if the initial volume of propane is 5.0 L and after 20 minutes of burning is 3.6 L? a. 7.0 ´ 10-2 L/min d. 22.2 L/min b. 14.3 L/min e. none of the above c. 4.5 ´ 10-2 L/min 5. What is the average rate of production of carbon dioxide for the system between 2.0 and 4.0 minutes, , if the concentration of carbon dioxide is 2.5 mol minutes and 7.2 mol/L after 4.0 minutes? a. 0.426 mol/(L·min) b. 1.18 mol/(L·min) c. 0.952 mol/(L·min) ____ ____ 6. What is the average rate of production of d. 2.35 mol/(L·min) e. 42.6 mol/(L·min) in the reaction of zinc with hydrochloric acid if after 30 s the volume of hydrogen is 20 mL, and after 1 minute, the volume is 62 mL? a. 62 mL/s d. either a or b b. 1.4 mL/s e. either b or c c. 62 mL/min 7. Within a reaction with 2 reactants and 2 products, the reaction rate can be measured with respect to a. all 4 substances d. only 1 of the substances b. 3 of the substances e. not enough information to determine c. 2 of the substances ____ 8. What property would be appropriate to measure the reaction rate in the following reaction: ? ____ a. change in conductivity d. change in pressure b. change in mass e. change in volume c. change in colour 9. What property would be appropriate to measure the reaction rate in the following reaction: a. change in conductivity d. change in pressure b. change in mass e. change in temperature c. change in colour ____ 10. What property would be appropriate to measure the reaction rate in the reaction between magnesium and hydrochloric acid? a. change in conductivity d. change in pressure b. change in mass e. change in volume c. change in colour ____ 11. What property would be appropriate to measure the reaction rate in the reaction, ____ 12. ____ 13. ____ 14. ____ 15. a. change in conductivity d. change in pressure b. change in mass e. change in volume c. change in colour Which of the following is not a factor that controls the rate of the reaction a. chemical nature of the reactants d. surface area b. concentration of the reactants e. temperature c. the number of products formed Generally, temperature affects the rate of a reaction in which of the following ways? a. increasing the temperature reduces the rate of the reaction b. decreasing the temperature decreases the rate of the reaction c. increasing the temperature increases the rate of the reaction d. both a and b are correct e. both b and c are correct The presence of a catalyst is thought to increase the rate of a reaction by a. changing the products that are formed in the reaction b. decreasing the enthalpy change of the reaction c. increasing the enthalpy change of the reaction d. decreasing the activation energy of the reaction e. increasing the activation energy of the reaction Which of the following is not usually considered a catalyst? a. water d. palladium b. platinum e. titanium(IV) oxide c. nickel ____ 16. A catalyst is only effective if it a. is present in large quantities b. is added before the reactants come in contact with each other c. is specific to the particular reaction d. is heated before it is added to the reaction e. all of the above ____ 17. According to the Rate Law, the rate (r) for the reaction a. [X] d. b. [Y] e. c. [products] ____ 18. The exponents determined for the overall rate law equation a. must be the same as the coefficients of the reaction b. can be multiplied together to determine the rate of the reaction c. can be added together to determine the rate of the reaction d. can be added together to determine the order of the reaction e. are independent of each other ____ 19. If for the reaction, of the reaction is which of the following? a. 1 b. 2 c. 3 ____ 20. If for the reaction a. b. c. d. e. d. 4 e. 5 , the rate law is determined to be , the order of the reaction is 0 increasing the concentration of Y will have no effect on the rate increasing the concentration of X will have no effect on the rate increasing the concentration of Y will increase the rate of the reaction there is no way to determine the value of k ____ 21. If for the reaction a. b. c. d. e. , the rate law is determined to be , the rate law is determined to be , doubling the concentration of Y will double the rate of the reaction halving the concentration of Y will double the rate of the reaction doubling the concentration of X will double the rate of the reaction halving the concentration of X will double the rate of the reaction only changes to the concentration of Y will affect the rate of the reaction ____ 22. The following graph represents a reaction of order must be proportiona a. 0 d. 3 b. 1 e. 4 c. 2 ____ 23. The following graph represents a reaction of order a. 0 b. 1 c. 2 ____ 24. The following graph represents a reaction of order d. 3 e. 4 a. 0 d. 3 b. 1 e. 4 c. 2 ____ 25. The following graph represents a reaction of order a. 0 d. 3 b. 1 e. not enough information c. 2 ____ 26. Nuclear decay, the change that occurs as a radioactive isotope breaks down, is a. a first order reaction d. varying depending on the isotope b. a second order reaction e. none of the above c. a third order reaction ____ 27. The half-life of a substance is a. half the time for the reactant to be completely consumed b. the time for the reaction to start c. the time for all of the reactant to be used up d. the time for half the reactant to be used up e. none of the above ____ 28. The half-life of all radioactive isotopes a. are all exactly the same b. are all less than 10 hours c. vary from milliseconds to billions of years d. very short e. cannot be determined ____ 29. Rates of reaction can be explained by a. atomic theory d. rate theory b. collision theory e. all of the above c. kinetic molecular theory ____ 30. Which of the following is not part of the collision theory? a. a chemical system consists of particles that are in constant motion b. a reaction must involve collisions of particles c. an effective collision has sufficient energy and correct orientation d. an ineffective collision can still cause a reaction e. the rate of the reaction depends on the frequency of effective collisions ____ 31. The following diagram represent a kinetic energy distribution at two temperatures. In comparing the two temperatures, it is obvious that a. d. b. e. it cannot be determined c. ____ 32. Ineffective collisions are collisions that involve particles a. without enough energy to react b. with the wrong orientation c. that rebound from the collision unchanged d. that cannot react e. all of the above ____ 33. The amount of energy required for a reaction to begin is known as a. enthalpy change d. kinetic energy b. reaction energy e. potential energy c. activation energy ____ 34. The activated complex a. is an unstable molecule b. has the maximum potential energy possible c. may continue on to produce products d. may revert to reactants e. all of the above ____ 35. In the following diagram, the letter which represents the position of the activated complex is: ____ 36. ____ 37. ____ 38. ____ 39. a. A d. D b. B e. E c. C If a reaction can be broken down into a reaction mechanism, then the steps of the reaction mechanism are known as a. stages of reaction d. elementary steps b. activated complexes e. primary equations c. reaction progress The number of particles that would usually "collide" in an elementary step are a. 1-2 d. unlimited b. 3-4 e. no particles need to collide c. 5-6 When a reaction is broken down into a number of elementary steps, this is known as a a. reaction progression d. reaction step b. reaction mechanism e. none of the above c. reaction pattern Reaction mechanisms a. are easily determined b. cannot be determined without knowing the enthalpy of the reaction c. are only 'best guesses' at the behaviour of molecules d. do not need to represent the whole reaction e. do not involve any reaction intermediates ____ 40. The rate determining step is a. the first step in a reaction mechanism b. the last step in a reaction mechanism c. the slowest step in a reaction mechanism d. the fastest step in a reaction mechanism e. the only step in a reaction mechanism ____ 41. Reaction intermediates are a. steps within a reaction mechanism b. the step that determines the rate of the reaction c. products formed in the overall equation d. products formed within some steps of the reaction mechanism e. none of the above ____ 42. Consider the above reaction mechanism. The rate-determining step of this reaction is a. elementary step 1 b. elementary step 2 c. elementary step 3 d. elementary steps 2 and 3 e. impossible to tell from this information ____ 43. Consider the above reaction mechanism. The reaction intermediates are formed in a. elementary step 1 b. elementary step 2 c. elementary step 3 d. elementary steps 1 and 2 e. impossible to tell from this information ____ 44. Consider the above reaction mechanism. The rate-law equation from this reaction would be a. d. b. c. e. impossible to tell from this information ____ 45. The theoretical effect of an increase in temperature can be explained in terms of collision theory because it affects I. the collision geometry involved in the reaction II. the total number of collisions that occur III. the fraction of collisions that are effective IV. the required activation energy for a reaction a. both I and IV d. both III and IV b. I, II and III are true e. II only c. both II and III ____ 46. The theoretical effect of an increase in the initial concentration of a reactant can be explained in terms of collision theory because it affects I. the collision geometry involved in the reaction II. the total number of collisions that occur III. the fraction of collisions that are effective IV. the required activation energy for a reaction a. both I and IV d. both III and IV b. I, II and III are true e. II only c. both II and III ____ 47. The theoretical effect of a catalyst can be explained in terms of collision theory because it affects I. the collision geometry involved in the reaction II. the total number of collisions that occur III. the fraction of collisions that are effective IV. the required activation energy for a reaction a. both I and IV d. both III and IV b. I, II and III are true e. II only c. both II and III ____ 48. The theoretical effect of the chemical nature of the reactants can be explained in terms of collision theory because it affects I. the collision geometry involved in the reaction II. the total number of collisions that occur III. the fraction of collisions that are effective IV. the required activation energy for a reaction a. both I and IV d. both III and IV b. I, II and III are true e. II only c. both II and III ____ 49. Which statement does not represent a rate? a. The speed of a car is 45 km/h. b. The half-life of an element is 12.4 h. c. A family consumes 4 L of milk every three days. d. It takes 15 min to walk to the store. e. A cat eats three cans of wet food per week. ____ 50. Which statement about the instantaneous rate of a reaction is not correct? a. The higher the rate, the greater is the slope of a line on a concentration-time graph. b. The instantaneous rate is the slope of the tangent to a line on a concentration-time graph. c. The instantaneous rate is the slope of the secant to a line on a concentration-time graph. d. The instantaneous rate decreases over time. e. All of these statements are correct ____ 52. In the following reaction, what is equal to the rate of production of NO gas? 4NH3(g) + 5O2(g) ® 4NO(g) + 6H2 O(g) a. the rate of production of NH3 gas b. one third the rate of production of water c. four fifths the rate of disappearance of O2 gas d. one quarter the rate of disappearance of NH3 gas e. six times the production of water vapour ____ 53. The initial rate of production of Br2 gas in the following reaction is 0.0750 mol/(L·s). What is the rate of loss of HBr gas? 4HBr(g) + O2(g) ® 2Br2(g) + 2H2 O(g) a. 0.0188 mol/(L·s) b. 0.0375 mol/(L·s) c. 0.0750 mol/(L·s) d. 0.150 mol/(L·s) e. 0.300 mol/(L·s) ____ 54. In the following reaction, butane is consumed at the rate of 0.0333 mol/(L·s). Determine the rate at which CO2 is produced. C4 H10(g) + ____ 55. ____ 56. ____ 57. ____ 58. ____ 59. ____ 60. O 2(g) ® 4CO2(g) + 5H2 O(g) a. 0.008 25 mol/(L·s) b. 0.0165 mol/(L·s) c. 0.0333 mol/(L·s) d. 0.0667 mol/(L·s) e. 0.133 mol/(L·s) Which factor will not affect the rate of the following reaction? Na(s) + AgNO3(aq) ® NaNO3(aq) + Ag(s) a. the addition of a catalyst b. an increase in pressure c. an increase in the concentration of AgNO3(aq) d. an increase in temperature e. all of these factors will have a strong effect on the rate of the reaction Over an interval of 1.00 s, the mass of propane changes by -5.87 g. What is the corresponding rate of production of carbon dioxide? 2C3 H8(g) + 9O2(g) ® 6CO2(g) + 6H2 O(g) a. 1.2 mol/s b. 0.60 mol/s c. 0.80 mol/s d. 0.20 mol/s e. 0.40 mol/s Which property cannot be used to measure the rate of the following reaction? Mg(s) + 2HCl(aq) ® MgCl2(aq) + H2(g) a. change in colour b. change in pH c. change in conductivity d. change in mass e. all of these properties could be used to measure the rate Which step of a reaction is the rate-determining step? a. the fastest step b. the first step c. the last step of the reaction mechanism d. the step with the greatest number of molecules e. the slowest step Which statement about the factors that affect reaction rates is false? a. Decreasing the concentrations of the reacting particles decreases the chance of collision. b. A collision with poor orientation requires a higher activation energy than a collision with optimum orientation. c. Increasing the pressure in a gaseous reaction increases the chance of collision. d. A reaction occurs every time particles of the reactants collide. e. Increasing the temperature increases the reaction rate. Given the following reaction mechanism, what is the equation for the overall reaction? ____ 61. ____ 62. ____ 63. ____ 64. ____ 65. 2A ® B + 2C (slow) B + C ® D + E (fast) C + D ® E + F (fast) a. 2A ® 2E + F b. 2A + B + 2C ® D + 2E + F c. 2A + 2C ® 2E + F d. 2A + B + 2C + D ® B + 2C + D + 2E + F e. 2A + C ® 2E + F Consider the following reaction mechanism. Changing the concentration of which substance(s) would have the most effect on the rate of the overall reaction? 2A ® B + 2C (slow) B + C ® D + E (fast) C + D ® E + F (fast) a. A b. A and C c. B d. D e. D and E Why does the rate of reaction increase with increasing temperature? a. The activation energy decreases as the temperature increases. b. Changing the temperature usually alters the reaction mechanism, similar to the effect of a catalyst. c. The change in temperature reduces the overall potential energy change between reactants and products. d. A greater proportion of all the molecules possess kinetic energy that is equal to or greater than the activation energy. e. Hotter molecules stick together better. Why does an increase in concentration increase the rate of reaction? a. Collisions become more effective. b. The number of collisions increases. c. The activation energy decreases. d. The average kinetic energy increases. e. When there are more molecules in the container, they all speed up. Which quantity does not increase when the temperature of a reaction system is raised? a. activation energy b. number of collisions c. number of effective collisions d. average kinetic energy of the particles e. all of the above increase To determine the rate of the following reaction, what physical property could be measured? H2(g) + I2(g) ® 2HI(g) a. change in concentration b. change in pH c. change in mass d. change in colour e. change in pressure Potential Energy Diagram ____ 66. Use the Potential Energy Diagram. What is shown by the letter A? a. the activation energy of the forward reaction b. the activation energy of the reverse reaction c. the transition state d. the heat of reaction e. the entropy of the reaction ____ 67. Use the Potential Energy Diagram. What is shown by the letter B? a. the activation energy of the forward reaction b. the activation energy of the reverse reaction c. the transition state d. the heat of reaction e. the entropy of the reaction ____ 68. Use the Potential Energy Diagram. What is shown by the letter C? a. the activation energy of the forward reaction b. the activation energy of the reverse reaction c. the transition state d. the heat of reaction e. the entropy of the reaction ____ 69. For an exothermic reaction, what does the activation energy of the reverse reaction equal? a. the activation energy of the forward reaction b. the heat of reaction minus the activation energy of the forward reaction c. the activation energy of the forward reaction minus the heat of reaction d. the heat of reaction plus the activation energy of the forward reaction e. the heat of reaction minus the activation energy of the reverse reaction ____ 70. The isotope has a half-life of 165 days. The isotope has a half-life of 330 days. Which statement about these two isotopes is false? a. One year represents slightly more than two half-lives of a sample of the calcium isotope. b. Almost all of 100 g of the calcium isotope will decay in one year’s time. c. 100 g of the vanadium isotope will decay to somewhat less than 50 g in a year. d. The calcium isotope decays at twice the rate of the vanadium isotope. e. All of these statements are true.