Potential Energy Diagrams Chemistry Worksheet
advertisement
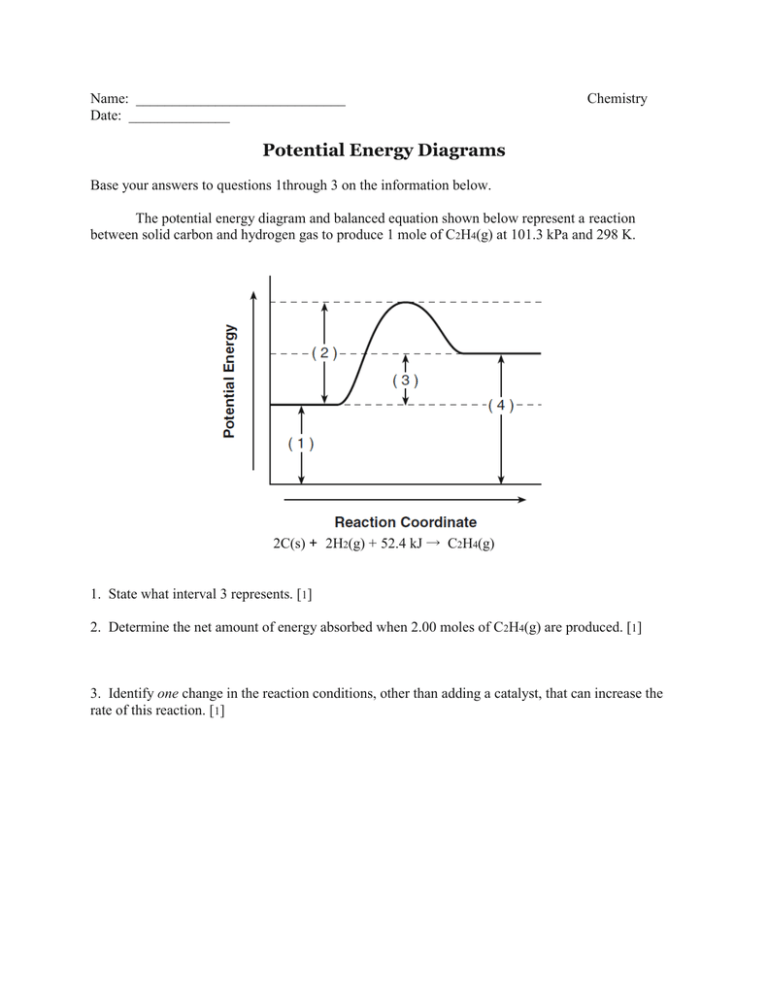
Name: _____________________________ Date: ______________ Chemistry Potential Energy Diagrams Base your answers to questions 1through 3 on the information below. The potential energy diagram and balanced equation shown below represent a reaction between solid carbon and hydrogen gas to produce 1 mole of C2H4(g) at 101.3 kPa and 298 K. 2C(s) + 2H2(g) + 52.4 kJ → C2H4(g) 1. State what interval 3 represents. [1] 2. Determine the net amount of energy absorbed when 2.00 moles of C2H4(g) are produced. [1] 3. Identify one change in the reaction conditions, other than adding a catalyst, that can increase the rate of this reaction. [1] 4. Given the potential energy diagram representing a reversible reaction: The activation energy for the reverse reaction is represented by (1) A + B (3) B + D (2) B + C (4) C + D 5. Given the equation and potential energy diagram representing a reaction: If each interval on the axis labeled “Potential Energy (kJ/mol)” represents 10. kJ/mol, what is the heat of reaction? (1) 60. kJ/mol (3) 30. kJ/mol (2) 20. kJ/mol (4) 40. kJ/mol Base your answers to questions 6 and 7 on the information below. The chemical reaction between methane and oxygen is represented by the potential energy diagram and balanced equation below. 6. Which potential energy interval in the diagram represents the activation energy of the forward reaction? [1] 7. Explain, in terms of collision theory, why a lower concentration of oxygen gas decreases the rate of this reaction. [1]