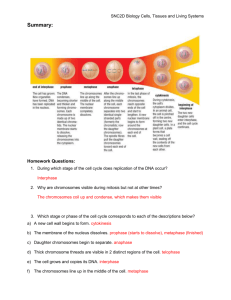
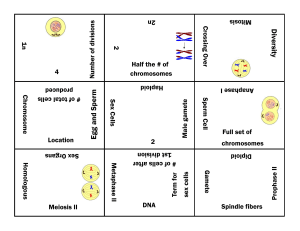
Cell and tissues: Structure and Function (21 questions in exam) 1. Which of these organelles is membranous? a) Centriole b) Ribosome c) Endoplasmic reticulum d) Cytoplasm 2. Which of these statements is correct? a) Cilia are microtubule-containing extensions of the cell membrane that increase the cell surface area to increase rates of absorption. b) Microvilli are microfilament-containing extensions of the cell wall that increase the cell surface area to increase rates of absorption. c) Cilia are microtubule-containing extensions of the cell wall that beat to in a synchronous fashion to move particulates along a luminal surface. d) Cilia are microtubule-containing extensions of the cell membrane that beat in a synchronous fashion to move particulates along a luminal surface. 3. The process of osmosis would explain the net movement of water out of a cell if the percentage of… A) water was 50% inside the cell and 50% outside the cell B) protein was 40% inside the cell and 45% outside the cell C) water was 65% inside the cell and 60% outside the cell D) water was 50% inside the cell and 55% outside the cell 4. Phagocytosis moves materials _____ a cell via _____. A) into ... facilitated diffusion B) into ... membranous vesicles C) into ... a transport protein D) into… osmosis 5. Which one of the following is NOT involved in active transport in some way? a) Active transport requires the expenditure of energy b) Active transport usually uses ATP c) Active transport usually moves solutes against their concentration gradient d) Active transport always moves solutes along their concentration gradient e) Active transport requires a protein carrier 6. The packaging of extracellular substances in vesicles at the cell surface for importing into the cell, is called? a) Exocytosis b) Facilitated diffusion c) Endocytosis d) Plasmodesmata e) Active transport 7. What is the specific name that is given to the process by which water crosses a selectively permeable membrane? a) b) c) d) e) Diffusion Passive transport Phagocytosis Pinocytosis Osmosis 8. What is a function of the smooth endoplasmic reticulum? a) Drug detoxification b) Synthesis lipids and steroids c) Carbohydrate metabolism d) All of the above 9. What is a function of the peroxisome? a) Breakdown of polysaccharides b) Production of proteins c) Breakdown of long chain fatty acids d) Digestion of glucose 10. What is NOT a constituent of the plasma membrane? a) Transmembrane protein b) Phosphoprotein bi-layer c) Glycolipid d) Cholesterol 11. What type of epithelia would be best suited for filtration and diffusion? a) Simple squamous b) Simple cuboidal c) Simple columnar d) Simply everywhere 12. What is the name of the mature bone cells housed in lacunae? a) Canaliculi b) Chondrocytes c) Lamellae d) Osteocytes 13. What type of tissue are glands? a) Lamina propria b) Epithelia c) Neural d) Specialised epithelia 14. Which of the following statements about muscle tissue is INCORRECT? a) Skeletal muscle fibres are long and cylindrical shape with many peripheral nuclei, that are striated. b) Cardiac muscle fibres are short, branched cells that are striated and connected by intercalated discs. c) Smooth muscle fibres are short, spindle shaped cells that are striated. d) Smooth muscle fibres are short, spindle shaped cells that lack striations. 15. Which of the following statements about nervous tissue is CORRECT? a) It consists of neurons and neuroligaments b) It contains neuroglia cells that conduct electrical signals. c) It consists of neurons that conduct electrical signals. d) It consists of neurons that support the conducting nerve cells. Cell division (5 questions in exam) 16. Which phase of cell division is the cytoplasm divided into daughter cells? a) S phase of interphase b) Prophase c) Telophase d) G1 phase of interphase 17. What phase of cell cycle are the organelles duplicated? a) G1 phase of interphase b) Prophase c) Cytokinesis d) G2 phase of telophase 18. During which phase are the DNA the most visible and condensed? a) Metaphase b) Prophase c) Anaphase d) Interphase 19. During which phase of mitosis do the centrioles position themselves at opposite poles of the cell? a) Telophase b) Metaphase c) Late prophase (prometaphase) d) Anaphase 20. What is the name of the structure that attaches to the kinetochores of the sister chromatids centromere? a) Centrioles b) Microtubules c) Centrosome d) Pili (singular: pilus) Human genetics (17 questions in exam) 21. To suffer from a recessive disorder, what is my allele combination for this trait? a) Homozygous dominant b) Homozygous recessive c) Heterozygous d) None of the above 22. Which of the following is a unique characteristic of meiosis (and not seen in mitosis? a) Crossing over at chiasma b) Daughter cells are gametes c) Daughter cells are haploid d) All of the above 23. What is the diploid number of chromosomes for humans? a) 23 b) 46 c) 22 d) 44 24. Recently in the gossip magazines Marge Simpson was accused of having an affair with Ned Flanders in the years between Lisa’s and Maggie’s birth. If this is in fact the case, Ned could be Maggie’s biological father? Knowing Maggie is type O blood and Marge is type A, what genotype must Ned be to have fathered Maggie? (construct punnet square if needed) a) IAIB b) IAIA c) IAi d) IBIB 25. Which of the following best summarises independent assortment? a) Each pair of homologous chromosomes can come for either mum or dad b) Each pair of homologous chromosomes aligns on the metaphase plate in one of two orientations irrelevant of the other pairs c) Each pair of homologous chromosomes aligns on the metaphase plate in the same orientation as all the other pairs d) Each pair of homologous chromosomes aligns on the metaphase plate and both chromosomes migrate to one pole 26. The pedigree below represents a family’s inheritance of Huntington’s disease. Based on your knowledge of pedigrees it can be determined that the disease is: a) Autosomal recessive b) Autosomal dominant c) Sex-linked recessive (X chromosome) d) Sex-linked (Y chromosome) 27. Tay-Sachs disease shows autosomal recessive inheritance. Parents of a newly diagnosed affected child are referred for genetic counselling. It would be correct to tell them that: a) The probability that their next child will be affected is 1:2. b) The probability of the older unaffected sister of the affected child is a carrier is 1:2. c) The fact that their last child was affected means that they could have another 3 children and they would not be affected. d) The parents are each a carrier. 28. What is the probability the parents in the previous question will have another child with Tay-Sachs: a) 0%. b) 25%. c) 50%. d) 75%. Microbiology (15 questions in exam) 29. What shape is the DNA in bacteria? a) Linear b) Bacteria do not carry DNA c) Circular d) Spiral 30. What is the term given to outbreaks that are on-going and always present in the community at a low frequency? a) Epidemiology b) Endemic c) Epidemic d) Pandemic 31. What is the term for a microorganism that doesn’t normally cause an infection, but under the right conditions such as when the host has weakened immune system, causes disease? a) Pathogenic b) Normal flora c) Transient flora d) Opportunistic 32. When observing the stages of disease development, which stage is associated with the period of exhibiting specific signs and symptoms? a) Prodromal b) Acute illness c) Malaise d) Incubation 33. If a person was subsequently infected with Lyme disease after being bitten by a tick, what mode of transmission would this be? a) Vector: mechanical transmission b) Vector: biological transmission c) Airborne transmission d) Droplet transmission 34. If a person was subsequently infected with cholera through the consumption of contaminated water, how would you classify the reservoir for that microbe? a) Animal b) Human c) Vector d) Non-living 35. What is the definition of a host and microbe relationship where the microorganism benefits however the host is harmed? a) Symbiosis b) Commensalism c) Mutualism d) Parasitism 36. What is the name given to microorganisms that persist long term in/on host and do not cause disease? a) Pathogenic b) Normal flora c) Transient flora d) Opportunistic Basic chemistry: including chemical reactions (17 questions in exam) 37. An element has an arrangement of 2-8-5. What is the name of this element? a) Nitrogen b) Phosphorus c) Boron d) Arsenic 38. Which of the following statements is true of non-metals; a) Will lose electron/s, form cations and bond ionically b) Will gain electron/s, form cations and bond ionically c) Will lose electron/s, form anions and bond covalently d) Will gain electron/s, form anions and bond covalently 39. Select the balance equation for the following reaction; ____ CoBr3 + ____ CaSO4 ____ CaBr2 + ____ Co2(SO4)3 a) 4CoBr3 + 5CaSO4 5CaBr2 + 2Co2(SO4)3 b) 2CoBr3 + 3CaSO4 3CaBr2 + Co2(SO4)3 c) 2CoBr3 + 3CaSO4 2CaBr2 +Co2(SO4)3 d) 3CoBr3 + 4CaSO4 4CaBr2 + 2Co2(SO4)3 40. The correct chemical formula for the covalent compound containing Phosphorus and chlorine is; a) b) c) d) P3Cl PCl2 PCl3 P2Cl3 41. What is the correct name for compound in the previous question (PCl3)? a) Phosphorus chloride b) Triphosphorus chloride c) Phosphorus trichloride d) Phosphide trichloride Gas laws (4 questions in exam) 42. Knowing the basic gas laws, gas properties are influenced by pressure, volume and temperature. However, why is temperature not a big influence on the gas properties of dissolved oxygen and carbon dioxide in our blood? a) Temperature is only influential on gases when extremely hot or cold b) Temperature is only influential when the gas/es are in the atmosphere c) Temperature is only influential in closed vessels d) Temperature is not influential on dissolved gases in the blood because the body maintains a narrow temperature range 43. Boyles law is one of the governing principles that define gas properties, it states when temperature remains constant…… a) The pressure of a gas is proportional to its volume b) The pressure of a gas is inversely proportional to its volume c) A gas behaves in an undefined manner d) A gas exerts the same pressure, irrelevant of its volume 44. A gas occupies 15 L at a pressure of 80 mm-Hg. What is the final volume when the pressure is increased to 1 atm? (1 atm = 760 mmHg) a) 2.3 L b) 142.5 L c) 1.6 L d) 14.3 L Solutions, dilutions and body fluids (9 questions in exam) 45. The substance ascorbic acid (C6H8O6) has a solubility of 33 g/100 mL in water at 40oC. Which one of the following solutions would be a saturated solution? a. adding 33 g of C6H8O6 to 100 mL of water at 40oC b. adding 50 g of C6H8O6 to 200 mL of water at 40oC c. adding 20 g of C6H8O6 to 50 mL of water at 40oC d. adding 5 g of C6H8O6 to 25 mL water at 40oC 46. A sodium hypochlorite solution (Milton’s) has a ratio concentration (strength) of 1:60. How many grams of sodium hypochlorite would be in 750 mL to make a 1:60 Milton’s solution? a) 12.5 g b) 60 g c) 0.08 g d) 8 g 47. What is the volume required to make a 5% glucose concentration that contains 3 grams of glucose? a) 60 mL b) 1500 mL c) 1667 mL d) 500 mL 48. What is the mass of NaCl required to prepare a 0.9% physiological saline solution with a volume of 400 mL? a) 3.6 g b) 0.225 g c) 4.44 g d) 2.25 g 49. You have a 10 mL hydrogen peroxide solution that has a concentration of 40 M. To bath infected wounds you need a 0.5 M hydrogen peroxide solution. How much water do you need to add to dilute it to a 0.5 M concentration. a) 0.8 L b) 0.08 L c) 0.008 L d) 0.0008 L 50. When NaCl is dissolved into water, to give Na+ and Cl- ions in solution, and the solution can now conduct electricity, it is an example of? a) an acid b) a base c) an electrolyte d) an heterogeneous mixture 51. A sodium hypochlorite solution (Milton’s) has a ratio concentration (strength) of 1:40. How many grams of sodium hypochlorite would be needed to make up 1000 mL of Milton’s solution? a) 25 g b) 250 g c) 40 g d) 4000 g Acids, bases, buffers and pH (8 questions in exam) 52. By definition a strong base is… a) A compound that strongly disassociates in water to give up hydroxide ions b) A compound that strongly disassociates in water to give up hydrogen ions c) A compound that strongly disassociates in water to give up a proton d) A compound that partially disassociates in water to give up hydroxide ions 53. What is the pH of a solution for which contains 1.7 x 10-3 M HCl? a) 2.77 pH b) 2.62 pH c) 1.7 pH d) 3 pH 54. A cleaning solution has a H+ concentration of 3.0 x 10 -8 what is the pH of this solution? a) 7.5 pH b) 6.5 pH c) 3 pH d) 8 pH 55. What is the balance of hydroxide ions (OH-) and hydrogen ions (H+) in pure water, that has a pH of 7? a) OH- higher than H+ b) H+ higher than OHc) OH- and H+ concentrations are equal to the number of H2O molecules d) OH- and H+ concentrations are equal 56. What is the general definition of a buffer solution? a) A solution that assists in altering the pH of either an acid or base b) A solution that resists a change in pH (when either an acid or base is added) c) A solution that resets the pH d) A solution that does not chemically react when either an acid or base is added Enzymes (4 questions in exam) 57. By definition an enzyme is a substance that; a) Partakes in the chemical reaction b) Lowers the activation energy of the reaction c) Can speed up many reactions (is not specific) d) Is used up in the reaction 58. An endothermic reaction is defined as; a) A reaction that absorbs heat energy b) A reaction that gives off heat energy c) A reaction that occurs only in high temperatures d) A reaction where the products have a higher energy state than the reactants 59. Which term most precisely describes the cellular process of building large molecules from smaller ones? a) metabolism b) catabolism c) anabolism d) catalysis 60. Which of the following statements about enzymes is NOT correct. a) Enzymes are proteins b) Human enzymes are denatured at close to freezing temperatures. c) Enzymes active sites bind substrate(s) that are converted to product(s) d) Enzymes are biological catalysts. ANSWERS 1. C 2. D 3. C 4. B 5. D 6. C 7. E 8. D 9. C 10. B 11. A 12. D 13. D 14. C 15. C 16. C 17. A 18. A 19. C 20. B 21. B 22. D 23. B 24. C 25. B 26. B 27. D 28. B 29. C 30. B 31. D 32. B 33. B 34. D 35. D 36. B 37. B 38. D 39. B 40. C 41. C 42. D 43. B 44. C 45. C 46. A 47. A 48. A 49. A 50. C 51. A 52. A 53. A 54. A 55. D 56. B 57. B 58. A 59. C 60. B