Chemistry Fundamentals Worksheet: Matter, Conversions, & More

Draw the difference between gas liquid and solid matter
Then write the difference between the three states of matter
List the diatomic molecules
Classify the following as an atom, molecule, or compound.
H
2
H
2
O
NaCl
Ar
C
6
H
12
O
6
Define a Homogeneous Mixture and give three examples of one
Define Heterogeneous Mixture and give 3 examples of one
_____________ Properties are those that we can measure without changing the basic identity of the substance (give examples)
_____________ Properties describes the way a substance may change or react to form another substance (give examples)
_____________ Properties characteristics of substances whose values do not depend on the amount of material chosen (give examples)
_____________ Properties characteristics of substances whose values do depend on the amount of material chosen
What is the difference between physical and chemical changes?
Metric conversions:
1km-
m
256cm
m
4378mm
m
37698m
cm
968
pm
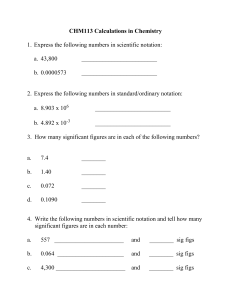
Convert to Scientific notation:
0.000135
18900000
Convert to standard notation:
9.5 x 10
7
38 x 10
-2
The density of magnesium is 1.89g/cm 3 . What is the volume of 275 g of this metal?
Write the conversions for Celsius, Fahrenheit, and Kelvin
Convert 87 degrees Fahrenheit to Celsius then to Kelvin.
Draw a diagram representing accuracy and then another representing precision
Write the correct amount of sig figs
412945
100045
23.04
0.3
098
0.000000349
1000.
1000
9.89000
Solve in the correct number of sig figs
0.085+0.062+0.14=
289-43.7
2.00 X 0.25
Dimensional Analysis
How many seconds in 18 hours?
5,400 in to mi
54 yards to mm
16 weeks to seconds