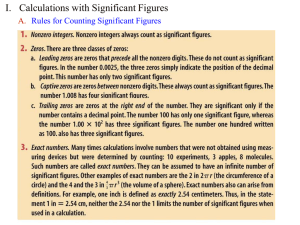
Unit 1 Review Name: Period:_____ Write each of the following
advertisement

Unit 1 Review Name:____________________ Period:_____ Write each of the following numbers in scientific notation. 1. 3405000000 3. 14 2. 0.00025 4. 950 Write each of the following numbers in standard form. 5. 8.2 x 105 7. 1.8 x 101 6. 4.7 x 10-3 8. 6.02 x 1023 Give the number of sig figs in each measurement. 9. 324 m 12. 0.054 cm 10. 150 kg 13. 1300.0 mL 11. 8032 s 14. 2001 C Perform the following calculations and report the answer using the proper number of sig figs. 15. 43.2 – (268.8/67.2) 17. 62.4 g + 79.27 g + 125.986 g 16. 13 cm x 120 cm x 23.8 cm 18. 120/(2.512 + 12.530) Perform the following conversions. Use significant figures in your answers. 19. 25.8 kg into pounds 21. 84 km into miles 20. 49987 minutes into years 22. 32 miles/gallon into kilometers/liter Perform the following temperature conversions. 23. 100C into kelvins 25. 398 K into Celsius 24. 450C into kelvins 26. 0 K into Celsius Find the percent error for each of the following measurements. 27. A student measures the length of an object to be 15.7 cm. The actual length of the object was 16.2 cm. 28. A group of students performed a lab and got 1.35 g of product. The actual amount that should have been obtained was 1.65 g. Perform the following calculations involving density. 29. A block of metal has a mass of 1.45 kg and displaces 542 mL of water when immersed. Calculate the density of the metal in g/mL. 30. Calculate the volume of 50.0 g of silver if the density of silver is 10.5 g/cm3. Graph the data listed below. Put volume on the y-axis and temperature on the x-axis. Be sure to include a title and to label both the x- and y-axis. Temperature (C) -15 0 21 48 80 Volume (mL) 4.4 4.6 5.0 5.4 5.9