Using Measurements Worksheet
advertisement
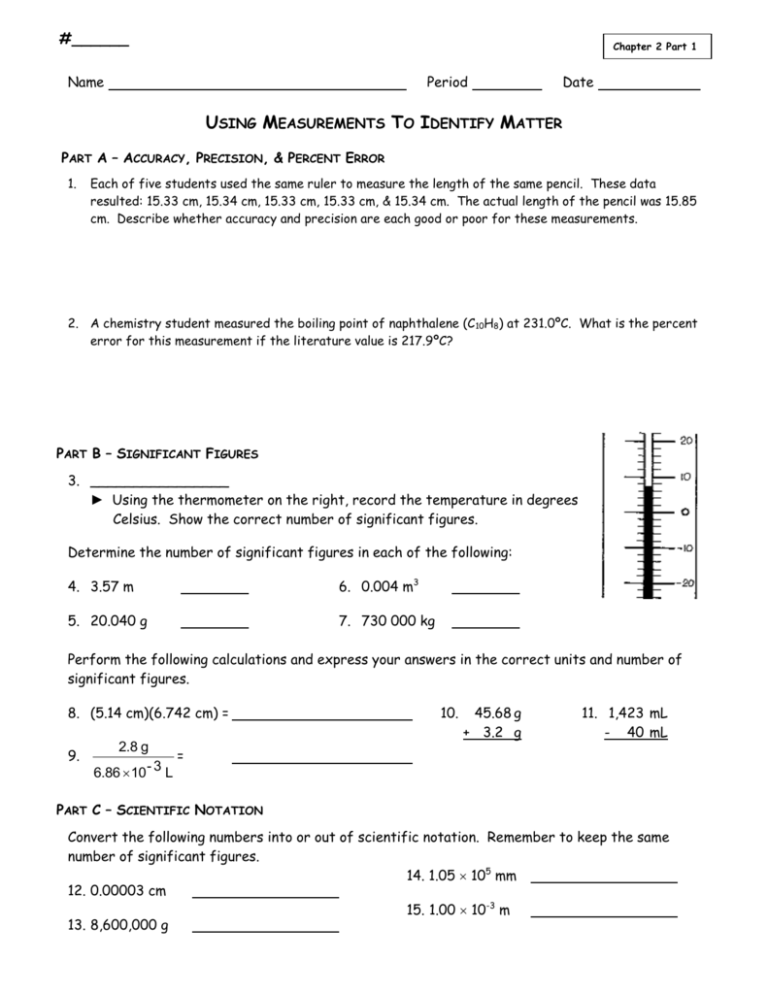
#______ Chapter 2 Part 1 Name Period Date USING MEASUREMENTS TO IDENTIFY MATTER PART A – ACCURACY, PRECISION, & PERCENT ERROR 1. Each of five students used the same ruler to measure the length of the same pencil. These data resulted: 15.33 cm, 15.34 cm, 15.33 cm, 15.33 cm, & 15.34 cm. The actual length of the pencil was 15.85 cm. Describe whether accuracy and precision are each good or poor for these measurements. 2. A chemistry student measured the boiling point of naphthalene (C 10H8) at 231.0ºC. What is the percent error for this measurement if the literature value is 217.9ºC? PART B – SIGNIFICANT FIGURES 3. ________________ ► Using the thermometer on the right, record the temperature in degrees Celsius. Show the correct number of significant figures. Determine the number of significant figures in each of the following: 4. 3.57 m 6. 0.004 m3 5. 20.040 g 7. 730 000 kg Perform the following calculations and express your answers in the correct units and number of significant figures. 8. (5.14 cm)(6.742 cm) = 9. 10. 45.68 g + 3.2 g 11. 1,423 mL - 40 mL 2.8 g = 6.86 10 - 3 L PART C – SCIENTIFIC NOTATION Convert the following numbers into or out of scientific notation. Remember to keep the same number of significant figures. 14. 1.05 105 mm 12. 0.00003 cm 15. 1.00 10-3 m 13. 8,600,000 g Using Measurements – Ch. 2 C. Johannesson CHEMISTRY TEST STUDY GUIDE UNIT 2 – Measurement Test Date: __________ Read over your notes, and rework your homework assignments. Reviewing your labs may also be helpful. The Periodic Table Reference Sheet will be provided. You are responsible for knowing the % error and density formulas. You will do AWESOME on this test if you can do the following things. Using Measurements Explain the difference between accuracy and precision. Calculate % error. Use the correct number of sig figs when making a measurement. Identify the number of significant figures in a number. Round off calculated answers to the correct number of sig figs. Convert between normal and scientific notation. Concert between Fahrenheit, Celsius, and kelvin. Distinguish between direct and inverse proportions. Units of Measurement Differentiate between a number and a quantity. Perform density calculations. Calculate density from the slope of a “Mass vs. Volume” graph. Unit Conversions Perform SI prefix conversions. Perform unit conversions using dimensional analysis. REMINDERS: You must show work for all calculations. Answers must include units & the correct # of sig figs. You must use dimensional analysis (grid method) for all unit conversions except simple SI prefix conversions. Ch. 4 – Uncertainty in Measurements CHEM