ASAP SCIENCE: science wars 3:37 mins)
WELCOME TO SCIENCE!
INTRO
Alternative to the above slide.
• Science STYLE –T-swizzle cover (asap science) 4:02
In this lesson:
• Safety
• Structure of an atom
• Electron configuration
• charge
SAFETY
Watch the video, list as many rules as you can from the video and why they are important.
What makes up an atom?
discuss
Atoms, Molecules, Elements and compounds
Atoms :
The smallest building block (besides sub-atomic particles)
Made up of a Nucleus (the centre) containing:
• Protons (positively charged)
• Neutrons (negatively charged).
Moving around the nucleus, like the earth does to the sun are Electrons, with
a negative charge.
Proton
Neutron
Electron
Think of an atom like a single brick of Lego,
everything is built from that brick onwards.
A ride that has only 2 seats can only
hold 2 people. You cannot have 5
people in 2 seats. The is the same when
we discuss the shells. Each shell has a
capacity.
Atoms
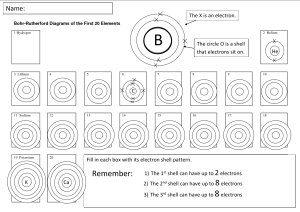
Each shell can only hold a certain number of electrons.
Shell 1- can only hold 2 electrons
Shell 2 – can only hold 8 electron
Shell 3- can only hold 8 electrons
There are more than 3 shells. You will be only learning the first 3 shells (first 20 elements on the periodic table.
THE PROCESS OF PLOTTING THE ELECTRONS INTO SHELLS IS CALLED ELECTRONIC
CONFIGURATION
Charge
(play from 2:30)
Electronic Configuration
worksheet
(OneNote under
E
Config. And bonding)
Kahoot / Quizziz