Colligative Properties Chemistry Worksheet
advertisement
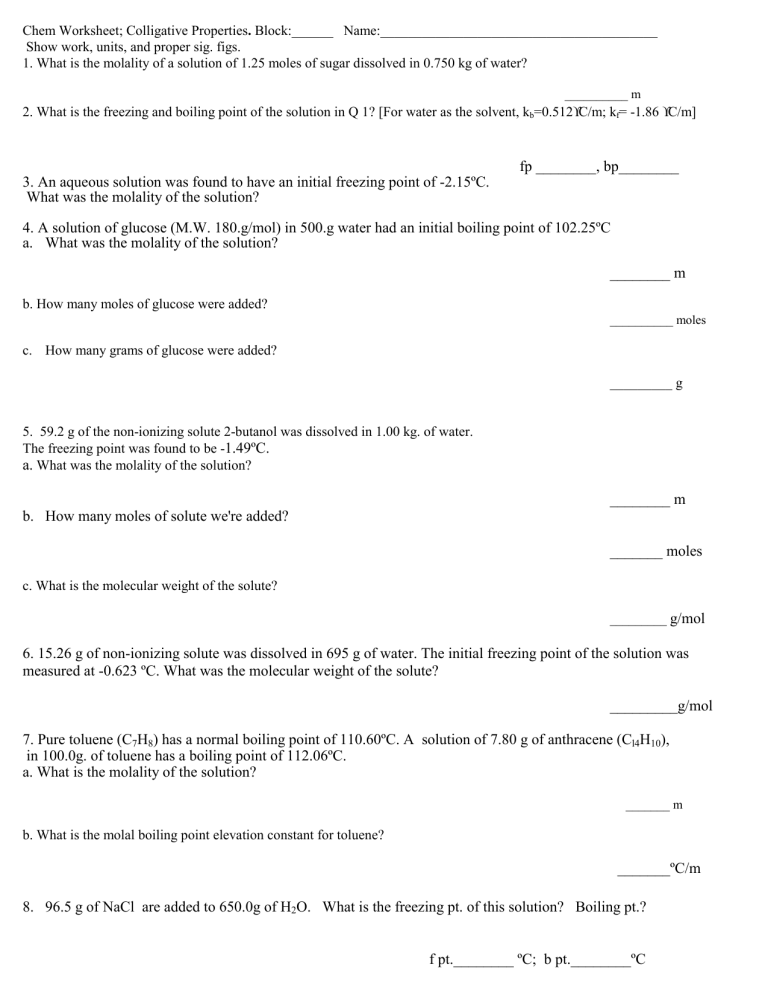
Chem Worksheet; Colligative Properties. Block:______ Name:________________________________________ Show work, units, and proper sig. figs. 1. What is the molality of a solution of 1.25 moles of sugar dissolved in 0.750 kg of water? __________ m 2. What is the freezing and boiling point of the solution in Q 1? [For water as the solvent, kb=0.512°C/m; kf= -1.86 °C/m] fp ________, bp________ 3. An aqueous solution was found to have an initial freezing point of -2.15ºC. What was the molality of the solution? 4. A solution of glucose (M.W. 180.g/mol) in 500.g water had an initial boiling point of 102.25ºC a. What was the molality of the solution? ________ m b. How many moles of glucose were added? __________ moles c. How many grams of glucose were added? _________ g 5. 59.2 g of the non-ionizing solute 2-butanol was dissolved in 1.00 kg. of water. The freezing point was found to be -1.49ºC. a. What was the molality of the solution? ________ m b. How many moles of solute we're added? _______ moles c. What is the molecular weight of the solute? _________ g/mol 6. 15.26 g of non-ionizing solute was dissolved in 695 g of water. The initial freezing point of the solution was measured at -0.623 ºC. What was the molecular weight of the solute? _________g/mol 7. Pure toluene (C7H8) has a normal boiling point of 110.60ºC. A solution of 7.80 g of anthracene (Cl4H10), in 100.0g. of toluene has a boiling point of 112.06ºC. a. What is the molality of the solution? _______ m b. What is the molal boiling point elevation constant for toluene? _______ºC/m 8. 96.5 g of NaCl are added to 650.0g of H2O. What is the freezing pt. of this solution? Boiling pt.? f pt.________ ºC; b pt.________ºC







