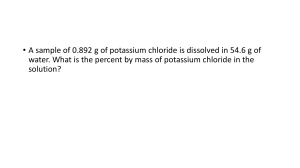Chapter 44 -
advertisement

Exam 2 – Chapter 15– Worksheet 2 Chapter 15: The Chemistry of Solutes and Solutions 1. Weight Percent to molarity. Concentrated nitric acid is a 70% solution of nitric acid, HNO 3, in water. The density of the solution is 1.41 g/ml at 25 celcius. What is the molarity of nitric acid in this solution? 2. Molarity to weight percent. Concentrated sulfuric acid has a density of 1.84 g/cm3 and is 18M. What is the weight percent of H2SO4 in the solution? 3. Molality. What is the molality of a solution made by dissolving 6.58 g NaCl in 250mL of water? What is the apparent molality? 4. Vapor Pressure Lowering and Mole Fraction. At 60°C the vapor pressure of pure water is 149.44 mm Hg and that above an aqueous sucrose (C12H22O12) solution is 119.55 mm Hg. Calculate the mole fraction of water, and the mass in grams of sucrose in the solution, if the mass of water is 150 g. 5. Freezing Point Depression, and Boiling Point Elevation. Some ethylene glycol, (HOCH2CH2OH) was added to your car’s cooling system along with 5.0 kg of water. (a) If the freezing point of the solution is -15°C, what mass (in grams) of of the glycol must have been added? (b) What is the boiling point of this mixture? K f =1.86 °C/m, Kb=0.512 °C/m. Exam 2 – Chapter 15– Worksheet 2 6. Apparent Molality, Boiling Point Elevation. The solubility of table salt in water at 100°C is 39.1 g/ 100 g of water. Calculate the boiling point of a saturated solution of NaCl. 7. Apparent Molality, Freezing Point Depression. A solution, prepared by dissolving 9.41 g NaHSO3 in 1.00 kg of water, freezes at -0.33°C. From these data, decide which of these equations is the correct expression for the dissociation of the salt: (a) NHSO3(aq) Na+ (aq) + HSO3- (aq) (b) NHSO3(aq) Na+ (aq) + H+ (aq) + SO32- (aq) 8. Molecular level descriptions.Using Lewis structure, draw a nanoscale diagram for the contents of a beaker containing a. water and ethanol b. Water and CCl4