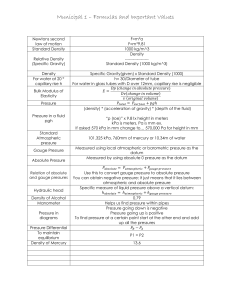
12/16/2015 The Kinetic Molecular Theory & Pressure Learning Goals: I will be able to explain the states of matter using the KMT. I will be able to explain pressure and atmospheric pressure. The Gas State Takes the shape and volume of the container Highly compressible Flows easily Random movement (called Brownian Motion after Brown’s work from early 1800s) 1 12/16/2015 The Kinetic Molecular Theory (KMT) All substances are composed of particles that are in constant, random motion. It is because of this motion that the scent of baking cookies will travel from the kitchen to your bedroom. Temperature Temperature is a measure of the average kinetic energy of the entities of a substance. So…as the particles move faster, the temperature increases. 2 12/16/2015 Pressure Is the force per unit area The SI unit is the Pascal (Pa) Atmospheric Pressure Is the pressure exerted by air on ALL objects. We use kilopascals (kPa) 3 12/16/2015 Some Standards… Standard Temperature & Pressure (STP) • 0°C • 101.325 kPa • Standard Ambient Temperature & Pressure (SATP) • 25°C • 101 kPa Atmospheric Pressure & Altitude The density of gases decreases as altitude increases, so atmospheric pressure decreases as altitude increases. 4 12/16/2015 Today’s Tasks Pg. 519 #1-2, 4-6 Pg. 546 #1, 3, 6 (for #1 use cross-multiplication 760 mm Hg = 101.325 kPa) 5