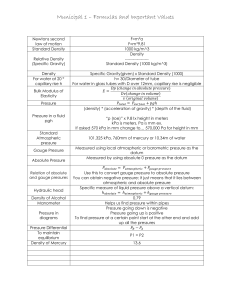
Republic of the Philippines TARLAC STATE UNIVERSITY College of Engineering and Technology CEP 372: FLUID MECHANICS LECTURE MODULE 2: PRINCIPLES OF HYDROSTATIC PRESSURES Prepared By: Engr. Karl Angelo G. Clarete 2.1 INTRODUCTION a.) PRESSURE: refers to the effect of a pressure acting against and distributed over a surface. They may be created by a solid, liquid or gas. It is the force per unit area exerted by a liquid or gas on a body or surface with the force acting at right angles to the surface uniformly in all directions. The pressure at any point in a fluid at rest is equal to its specific weight (γ) multiplied by the vertical depth (h) of the point from the surface, or 𝒑 = 𝜸𝒉 where: p – pressure (Pa, psi, psf) 𝛾 – specific or unit weight (N/m3, lb/ft3) h – height or depth (m, ft) If F represents the total force on an area A, and dF is the elemental force on an elemental area dA, the intensity of pressure is, 𝒑= 𝒅𝑭 𝒅𝑨 b.) PASCAL’S LAW: developed by French mathematician Blaise Pascal. States that, “The pressure on a fluid is equal in all directions and in all parts of the container.” Note: If the pressure is uniform over the area A, then 𝒑= 𝑭 𝑨 Otherwise this formula gives only the average pressure 𝐬𝐢𝐧 𝜽 = 𝑨𝑨𝑩𝑬𝑭 𝑨𝑨𝑩𝑪𝑫 where: 𝐜𝐨𝐬 𝜽 = 𝑨𝑪𝑫𝑬𝑭 𝑨𝑨𝑩𝑪𝑫 p – pressure (Pa, psi, psf) F – force (N, lb) A – cross sectional area (m2, ft2) Page | 1 of mercury 1 inch in height at the standard + acceleration of gravity. 𝑭𝒙 = 𝟎 Millibar is 1/1000th of a bar and is the amount of 𝑭𝒙 − 𝑭𝒚 𝐬𝐢𝐧 𝜽 = 𝟎 𝑷𝒙 𝑨𝑨𝑩𝑬𝑭 − 𝑷𝒚 𝑨𝑨𝑩𝑪𝑫 𝑨𝑨𝑩𝑬𝑭 = 𝟎 𝑨𝑨𝑩𝑪𝑫 one centimeter, in one second. Millibar values used in meteorology ranges from about 100 to 1050. At sea level, standard air pressure in millibars is 1013.2. 𝑷𝒙 = 𝑷𝒚 + force it takes to move an object weighing a gram, 2.3 GAGE PRESSURE (RELATIVE 𝑭𝒚 = 𝟎 PRESSURE) a.) GAGE PRESSURE: are pressures above or below 𝑭𝒛 − 𝑭𝒚 𝐜𝐨𝐬 𝜽 = 𝟎 𝑷𝒛 𝑨𝑪𝑫𝑬𝑭 − 𝑷𝒚 𝑨𝑨𝑩𝑪𝑫 𝑨𝑪𝑫𝑬𝑭 = 𝟎 𝑨𝑨𝑩𝑪𝑫 𝑷𝒛 = 𝑷𝒚 ∴ 𝑷𝒙 = 𝑷𝒚 = 𝑷𝒛 ∴ The pressure on a fluid is equal in all directions 2.2 ATMOSPHERIC PRESSURE a.) ATMOSPHERIC PRESSURE: is the pressure at any one point on the earth’s surface from the weight of the air above it. Refers to the prevailing pressure in the air around us. a.1.) STANDARD ATMOSPHERIC PRESSURE 𝑷𝒂𝒕𝒎 = 𝟏 𝒂𝒕𝒎 𝑷𝒂𝒕𝒎 = 𝟏𝟎𝟏. 𝟑𝟐𝟓 𝒌𝑷𝒂 𝑷𝒂𝒕𝒎 = 𝟏𝟒. 𝟕 𝒑𝒔𝒊 𝑷𝒂𝒕𝒎 = 𝟐𝟏𝟔𝟔 𝒍𝒃/𝒇𝒕𝟐 𝑷𝒂𝒕𝒎 = 𝟕𝟔 𝒄𝒎 𝒐𝒇 𝑯𝒈 𝑷𝒂𝒕𝒎 = 𝟐𝟗. 𝟗 𝒊𝒏 𝒐𝒇 𝑯𝒈 𝑷𝒂𝒕𝒎 = 𝟏𝟎𝟏𝟑 𝒎𝒊𝒍𝒍𝒊𝒃𝒂𝒓𝒔 Note: Centimeters of Mercury is a small pressure unit which represents the pressure pushing down due to gravity of any volume of liquid mercury which is 1 the atmosphere and can be measured by pressure gauges or manometers. b.) VACUUM PRESSURE: A space that has all matter removed from it. Can also be described as a region of space where the pressure is less than the normal atmospheric pressure of 760 mm (29.9 in) of mercury. It is the pressure below the atmospheric pressure and is used when the gage pressure is negative. 2.4 ABSOLUTE PRESSURE a.) ABSOLUTE PRESSURE: Is the pressure above absolute zero. Absolute pressure can never be negative and absolute zero is obtained if all air is removed. It is the lowest possible pressure attainable. 𝑷𝒂𝒃𝒔 = 𝑷𝒈𝒂𝒈𝒆 + 𝑷𝒂𝒕𝒎 where: 𝑃 – absolute pressure (Pa, psi, psf) 𝑃 – gage pressure (Pa, psi, psf) 𝑃 – atmospheric pressure (Pa, psi, psf) cm high. Inch of mercury is a non-SI unit of measurement for pressure. It is used for barometric pressure in weather reports, refrigeration and aviation in the United States. It is the pressure exerted by a column Page | 2 2.5 EQUIPMENT FOR PRESSURE MEASUREMENT a.) MERCURY BAROMETER: is an accurate and relatively simple way to measure changes in c.) MANOMETER: is a tube, usually bent in a form of U, containing a liquid of known specific gravity, the surface of which moves proportionally to changes of pressure. It is used to measure pressure. atmospheric pressure. At sea level, the weight of the atmosphere forces mercury 760 mm (29.9 in) goes up a calibrated glass tube. Higher elevations yield lower readings because the atmosphere is less dense there, and the thinner air exerts less pressure on the mercury. 2.6 TYPES OF MANOMETER a.) OPEN TYPE: has an atmospheric surface in one leg or two legs and is capable of measuring gage pressures. b.) ANEROID BAROMETER: In an aneroid barometer, a partially evacuated metal drum expands or contracts in response to changes in air pressure. A series of levers and springs translates the up and down movement of the drum top into the circular motion of the pointers along the aneroid barometer’s face. b.) DIFFERENTIAL TYPE: without an atmospheric surface and capable of measuring only differences of pressure. Page | 3 c.) PIEZOMETER: the simplest form of open at rest, all forces acting upon it must be in manometer. It is a tube tapped into a wall of a equilibrium. container or conduit for the purpose of measuring FLS pressure. The fluid in the container or conduit rises in this tube to form a free surface. c.1.) LIMITATIONS OF PIEZOMETER Note: Large pressures in the lighter liquids require FREE LIQUID SURFACE (FLS): refers to a liquid long tubes. surface subject to zero gage pressure or with Gas pressures cannot be measured because atmospheric pressure only. gas cannot form a free surface. 2.7 STEPS IN SOLVING MANOMETER PROBLEMS 1.) Decide on the fluid in feet or meter, of which the heads are to be expressed, (water is the most With reference to illustration shown, 𝑾 = 𝜸𝑽 𝑾 = 𝜸(𝒂𝑳) + 𝑭𝒙 = 𝟎 advisable base fluid). 2.) Starting from an end point, number in order, the interface of different fluids. 3.) Identify points of equal pressure (taking into account that for a homogeneous fluid at rest, the pressure along the same horizontal plane are equal). Label these points with the same number. 4.) Proceed from level to level, adding (if going down) or subtracting (if going up) pressure heads as the elevation decreases or increases, respectively with due regard of the specific gravity of the fluids. 𝑭𝟐 − 𝑭𝟏 = 𝑾𝒔𝒊𝒏𝜽 𝑷𝟐 𝒂 − 𝑷𝟏 𝒂 = 𝜸(𝒂𝑳)𝒔𝒊𝒏𝜽 𝑷𝟐 − 𝑷𝟏 = 𝜸𝑳𝒔𝒊𝒏𝜽 𝒃𝒖𝒕 𝑳𝒔𝒊𝒏𝜽 = 𝒉 𝑷𝟐 − 𝑷𝟏 = 𝜸𝒉 b.) PRESSURE POINTS LIE ON THE SAME ELEVATION: Considering that two points (1 and 2) lie on the same elevation, such that ℎ = 0, then: FLS 2.8 VARIATIONS IN PRESSURE a.) PRESSURE POINTS DO NOT LIE ON THE SAME ELEVATION: Considering any two points (1 and 2), whose difference in elevation is ℎ, to lie in the ends of an elementary prism having a cross sectional area 𝑎 and a length of 𝐿. Since the prism is Page | 4 With reference to illustration shown, 𝛾 – specific or unit weight (N/m3, lb/ft3) a.1.) TO CONVERT PRESSURE HEAD OF + LIQUID A TO LIQUID B: 𝒔𝑨 𝝆𝑨 𝜸𝑨 𝒉𝑩 = 𝒉𝑨 𝑜𝑟 𝒉𝑩 = 𝒉𝑨 𝑜𝑟 𝒉𝑩 = 𝒉𝑨 𝒔𝑩 𝝆𝑩 𝜸𝑩 𝑭𝒙 = 𝟎 𝑭𝟐 − 𝑭𝟏 = 𝟎 𝑷𝟐 𝒂 − 𝑷 𝟏 𝒂 = 𝟎 (𝒂)(𝑷𝟐 − 𝑷𝟏 ) = 𝟎 𝑷𝟐 − 𝑷𝟏 = 𝟎 𝑷𝟐 = 𝑷𝟏 c.) PRESSURE BELOW LAYERS OF DIFFERENT LIQUIDS: Consider the tank shown to be filled with liquids of different densities and with air at the top under a gage pressure of 𝑝 , the pressure at the bottom of the tank is: a.2.) TO CONVERT PRESSURE HEAD OF ANY LIQUID TO WATER: 𝒉𝒘𝒂𝒕𝒆𝒓 𝒔𝒘𝒂𝒕𝒆𝒓 = 𝒉𝒍𝒊𝒒𝒖𝒊𝒅 𝒔𝒍𝒊𝒒𝒖𝒊𝒅 𝒔𝒘𝒂𝒕𝒆𝒓 = 𝟏 𝒉𝒘𝒂𝒕𝒆𝒓 = 𝒉𝒍𝒊𝒒𝒖𝒊𝒅 𝒔𝒍𝒊𝒒𝒖𝒊𝒅 2.10 LIQUID PRESSURE IN A HYDRAULIC SYSTEM In a hydraulic system, fluid is confined to two chambers. Each chamber has a piston that is free to move. 𝑷𝒃𝒐𝒕𝒕𝒐𝒎 = 𝑷 𝑷= 𝑷𝒃𝒐𝒕𝒕𝒐𝒎 = 𝑷𝒂𝒊𝒓 + 𝜸𝟏 𝒉𝟏 + 𝜸𝟐 𝒉𝟐 + 𝜸𝟑 𝒉𝟑 2.9 PRESSURE HEAD a.) PRESSURE HEAD: Is the height ℎ of a column of homogenous liquid of unit weight γ that will produce an intensity of pressure 𝑃. From the formula for pressure, 𝑷 = 𝜸𝒉 𝑷 𝒉= 𝜸 where: 𝑷𝟏 = 𝑭 𝑨 𝑭𝟏 𝑭𝟐 𝑎𝑛𝑑 𝑷𝟐 = 𝑨𝟏 𝑨𝟐 According to Pascal’s Law: 𝑷𝟏 = 𝑷𝟐 𝑭𝟏 𝑭𝟐 = 𝑨𝟏 𝑨𝟐 where: 𝑃𝑖𝑠𝑡𝑜𝑛 1 − 𝑀𝐴𝑆𝑇𝐸𝑅 𝐶𝑌𝐿𝐼𝑁𝐷𝐸𝑅 𝑃𝑖𝑠𝑡𝑜𝑛 2 − 𝑆𝐿𝐴𝑉𝐸 𝐶𝑌𝐿𝐼𝑁𝐷𝐸𝑅 h – pressure head (m, ft) p – pressure (Pa, psi, psf) Page | 5 2.11 SAMPLE PROBLEMS EXAMPLE #1: In a depth of liquid of 1 m causes a pressure of 7 kPa, what is the specific gravity of the liquid? EXAMPLE #7: In the figure shown, if the atmospheric pressure is 101.03 kPa and the absolute pressure at the bottom of the tank is 231.3 kPa, what is the specific gravity of olive oil? EXAMPLE #2: What is the pressure in kPa 12.5 m below the ocean? Use specific gravity = 1.03 for ocean water. EXAMPLE #3: If the pressure 23 meter below a liquid is 338.445 kPa, determine its (a) unit weight in kN/m3, (b) mass density in kg/m3, and (c) specific gravity. EXAMPLE #4: If the pressure at a point in the ocean is 60 kPa, what is the pressure in kPa 27 meters below this point? Use specific gravity = 1.03 for ocean water. EXAMPLE #5: If the pressure in the air space above an oil (s = 0.75) surface in a closed tank is 115 kPa absolute, what is the gage pressure in kPa 2 m below the surface? "Motivation is what gets you started. Habit is what keeps you going." – Jim Ryun EXAMPLE #6: A pressure gage 6 m above the bottom of the tank containing a liquid reads 90 kPa. Another gage height 4 m reads 103 kPa. Determine the specific weight of the liquid in kN/m3. Page | 6