SOLUTIONS REVIEW
advertisement

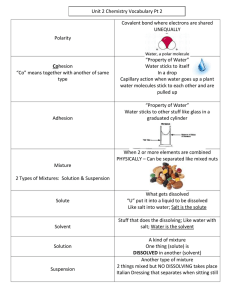
SOLUTIONS REVIEW Solvent: the liquid part of the solution, in an aqueous solution it is water. Solute: what is dissolved into the solvent. UNSATURATED: the solvent can dissolve more solute. Not all the water molecules are involves in molecule to ion attractions. SATURATED: the solvent is full – it cannot dissolve any more solute. All the water molecules are involved in molecule to ion attractions. SUPERSATURATED: the solvent is full and excess solute precipitates (salt solutes) or bubbles out of solution (gas solutes). Dissolving is EQUILIBRIUM; at saturation the rate of dissolving is equal to the rate of precipitation. - at saturation solutions obey Le Chatelier’s Principle, for solutions the temperature is most important as solids do NOT affect equilibrium. o The system will try to prevent its equilibrium quantities from changing. o If you ADD to one side, the reaction runs to the opposite side to get rid of it in order to preserve the equilibrium that existed. o If you REMOVE or DILUTE something from one side, the reaction runs to that side to replace it. o If you heat an endothermic reaction, you push it forward (endothermic salts dissolve) o If you heat an exothermic reaction, you reverse it (gasses bubble out). EXAMPLE, the dissolving of the salt sodium nitrate is endothermic, it absorbs heat. Adding heat shifts the reaction away to the right, salt dissolves. NaNO3(s) + HEAT Na+(aq) + NO3-(aq) Cooling – removing heat shifts the reaction to the left, the salt precipitates EXAMPLE: the dissolving of ammonia gas is exothermic, releases heat Adding heat shifts the reaction away to the left, gas bubbles out. Decreasing pressure will push the reaction to the most moles of gas NH3(g) NH3(aq) + HEAT Cooling – removing heat shifts the reaction to the right, the gas dissolves. Increasing pressure will drive the reaction to the least moles of gas. DILUTION: adding water will decrease the concentration of salt ions(reducing the concentration, similar to removing some ions), the reaction will compensate by dissociating to replace them. Adding water reduces the concentration of ions (increases aqueous phase), the reaction runs to the product side to increase the concentration of ions. The salt dissolves. NaNO3(s) + HEAT NO3- (aq) + Na-(aq) Removing (evaporating) water increases the concentration of ions, the reaction runs left to get the concentration back down, the salt precipitates.