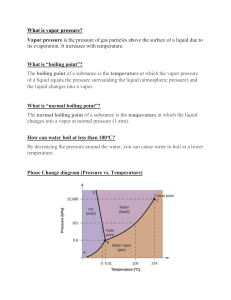
All About solutions solutions A SOLUTION is a homogeneous mixture. It is a mixture when one substance is dissolved in another so the properties are the same throughout. A solutions is composed of a: - SOLUTE: the substance that is dissolved in the solution solute AND - SOLVENT: the substance that does the dissolving solvent About solutions An UNSATURATED SOLUTION is a solution that contains less than the maximum amount of solute that is capable of being dissolved. A SATURATED SOLUTION is a solutions that contains the maximum amount of solute that is capable of being dissolved. A SUPERSATURATED solution is a solution that contains more dissolved substance than a saturated solution. Example, carbonated water. About solutions Soluble and insoluble The SOLUBILITY is the ability of a substance to dissolve in a solvent and form a solution. SOLUBLE – A substance that can be dissolved INSOLUBLE – a substance that CANNOT be dissolved Coffee Sand Sugar Wood Concentration The CONCENTRATION refers to how much solute is in a solution. Water is the universal solvent because it can dissolve more substances than any other liquid. Understanding matter To Understand Matter We Can Describe It: - Physical Properties - Color - Mass - Boiling point - Melting point - Size and shape - Chemical Properties - Reactivity - Flammability - Heat of Combustion - Toxicity - Ability to Oxidize