Nuclear Notation: Mass Number, Atomic Number, Isotopes
advertisement
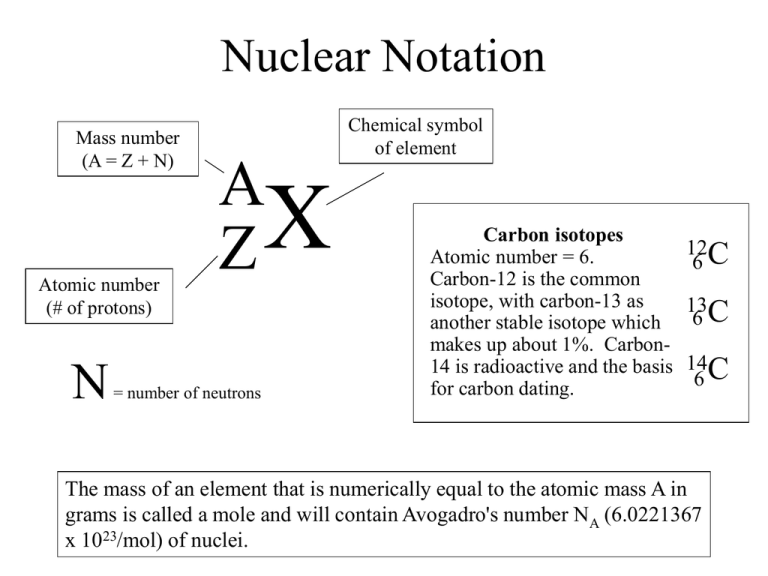
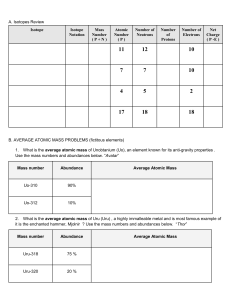
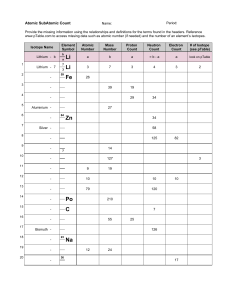
Nuclear Notation Mass number (A = Z + N) Atomic number (# of protons) N Chemical symbol of element A X Z = number of neutrons Carbon isotopes Atomic number = 6. Carbon-12 is the common isotope, with carbon-13 as another stable isotope which makes up about 1%. Carbon14 is radioactive and the basis for carbon dating. 12C 6 13C 6 14C 6 The mass of an element that is numerically equal to the atomic mass A in grams is called a mole and will contain Avogadro's number NA (6.0221367 x 1023/mol) of nuclei.