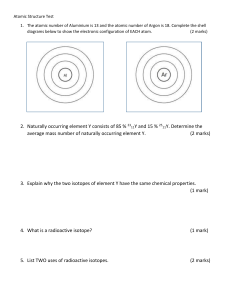
What is Relative Atomic Mass? Relative Atomic Mass can be written as RAM or Ar. In a naturally occurring sample of Chlorine, 75% of the atoms will have a mass number of 35, 25% will have a mass number of 37. The average mass number for the sample will be [(75 x 35) + (25 x 37)] ÷100 = 35·5 The average mass number is called the Relative Atomic Mass (RAM). In this way, the relative atomic mass of any element can be calculated, given the percentage of each isotope. The mass number of any one atom will be a whole number. the average mass number for all of the isotopes of that element. The RAM is written in green above each element in the periodic table. The relative atomic mass is