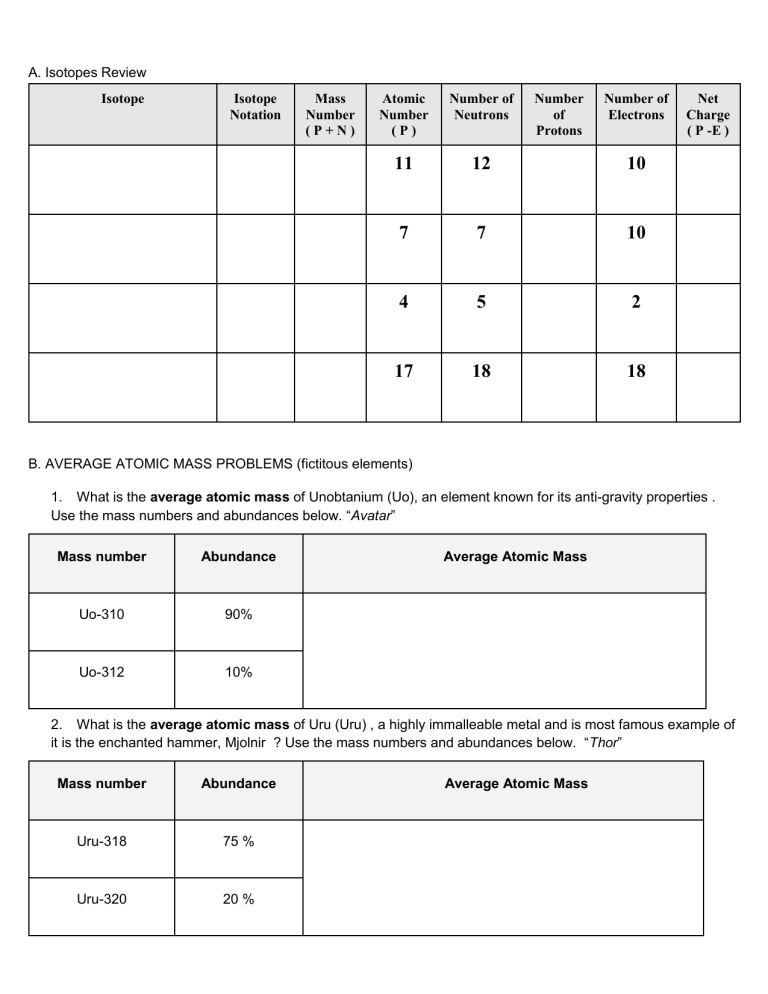
A. Isotopes Review Isotope Isotope Notation Mass Number (P+N) Atomic Number (P) Number of Neutrons Number of Protons Number of Electrons 11 12 10 7 7 10 4 5 2 17 18 18 Net Charge ( P -E ) B. AVERAGE ATOMIC MASS PROBLEMS (fictitous elements) 1. What is the average atomic mass of Unobtanium (Uo), an element known for its anti-gravity properties . Use the mass numbers and abundances below. “Avatar” Mass number Abundance Uo-310 90% Uo-312 10% Average Atomic Mass 2. What is the average atomic mass of Uru (Uru) , a highly immalleable metal and is most famous example of it is the enchanted hammer, Mjolnir ? Use the mass numbers and abundances below. “Thor” Mass number Abundance Uru-318 75 % Uru-320 20 % Average Atomic Mass Uru-325 5%