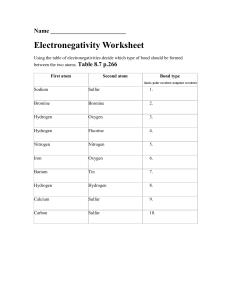
TYPES OF BONDING & COMPOUNDS WORKSHEET I. Fill in the blanks below and use your notes on Chemical Bonding to help. 1. There are three types of bonds. Ionic bonds are formed between a ______________________ element and a ______________________ element. ______________________ bonds are formed between 2 nonmetals and lastly there are ______________________ bonds that are formed between ______________________. 2. Ionic bonds form a ______________________ of repeating positive and negative ions while ______________________ bonds ______________________ their electrons forming molecules which can be ______________________ when e- are shared equally or can be ______________________ when e- are shared unequally. 3. Most ionic compounds have ______________________ melting and boiling point temperatures while covalent compounds have ______________________ melting and boiling point temperatures. 4. Hydrogen is one of the seven ______________________ elements that exist in nature as two atoms of that element bonded together and are ______________________ because it shares its electrons equally. II. Identify the type of bond [ionic (I), covalent (C), or metallic (M) bond] that would be formed between the following pairs of elements. III. IV. 1) potassium and iodine __________ 6) carbon and fluorine __________ 2) phosphorus and oxygen __________ 7) silver and copper __________ 3) strontium and chlorine __________ 8) oxygen and hydrogen __________ 4) tin and lead __________ 9) chlorine and bromine __________ 5) nitrogen and bromine __________ 10) hydrogen and hydrogen __________ Identify the type of compound [ionic (I), covalent (C), or metallic (M) bond] for the following substances. 11) C6H6 __________ 16) gold bracelet __________ 12) aluminum foil __________ 17) O2 __________ 13) Li2S __________ 18) NH3 __________ 14) C2Cl6O __________ 19) CuF3 __________ 15) Mg3(PO4)2 __________ 20) SO2 __________ Identify if the following pairs of elements would form a nonpolar covalent (NPC) or polar covalent (PC) bond. 21) oxygen and hydrogen __________ 35) iodine and fluorine __________ 22) carbon and fluorine __________ 26) sulfur and oxygen __________ 23) nitrogen and nitrogen __________ 27) fluorine and fluorine __________ 24) oxygen and bromine __________ 28) nitrogen and hydrogen __________