Electromagnetic Radiation (EMR) Electromagnetic radiation (EMR): EM spectrum:
advertisement

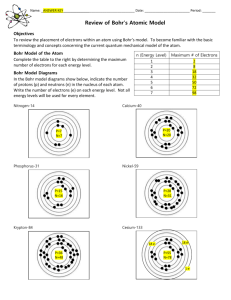
Electromagnetic Radiation (EMR) Electromagnetic radiation (EMR): EM spectrum: Parts of a wave: Waves have 3 primary characteristics: 1. 2. 3. The wavelength (λ) and the frequency (ν) are inversely proportional:_______________________ _________________________________________________________________________ Wavelength and frequency can be inverconverted λν = c λ= ν= c= 𝑐 ν=λ Photons: ____________ - tiny paricle of electromagnetic radiation – a bundle of light energy ____________ - electrons are at their lowest energy state in an atim ____________ - electrons have absorbed energy by “jumping” up to a higher energy state in an atom Light can travel as particles called photons These particles absorb and emit light only in specific quanta ("packets" of energy) Atomic Line Spectrum of Excited Hydrogen Atoms __________________________ An object can gain or lose energy by absorbing or emitting radiant energy in_________ larger energy jumps produce shorter wavelegth (more energetic) light (see below) The transfer of energy is quantized and can only occur in discrete units called quanta. ΔE = hν = ℎ𝑐 𝜆 ΔE = Planck’s constant: h = 6.626 x 10-34 J · 𝑠 ν= λ= Say what? Einstein’s experiment demonstrates the particle nature of light Classical theory said that the E (energy) of ejected electrons should increase with an increase in light intensity – this was not observed! What happened: No electrons were observed until light of a certain minimum E is used (threshold amount) Number of e- ejected depends on light intensity Line Spectra of Excited Atoms: Excited Atoms emit light of only ____________ wavelengths The wavelengths of light depend on the element Example: Atomic Spectrum of Hydrogen: _________________: Contains ____________ the wavelengths of light _________________: (discrete) spectrum: contains only some of the wavelengths of light The diagram above present evidence for discrete energy levels about a nucleus. Electrons can only be found in certain energy levels with certain energies. Niels Bohr’s greatest contribution to science was building a simple model of the atom. It was based on an understanding of the BRIGHT LINE SPECTRA of excited atoms. Bohr Model of the Atom: _____________________: The lowest possible energy state for an atom (n = 1) Higher energy electrons are in outer shells and easier to remove because they are more shielded from the positive nucleus by the inner shell electrons. Bohr’s model was incorrect - replaced by _______________________________ e- cannot be exactly located – location based on ____________________ QUANTUM MECHANICS: Major Players (including Bohr): deBroglie (1924) – proposed that all moving objects have wave properties Schrödinger applied the idea of e- behaving as a wave to the problem of electrons in atoms Wave equation predicts: Heisenberg solved problem of defining the nature of electrons in atoms Cannot simultaneously define the position and momentum (m · v) of an electron We define e- energy exactly but accept the limitation that we do not know exact position Failure of the Bohr Model: The Bohr model of the atom paved the way for Quantum Mechanical Theory, but current theory is in ______ way derived from the Bohr model of the atom. Although is it a useful model to draw atoms, the Bohr model of the atom was fundamentally incorrect – atoms do ______ move in circular orbits about the nucleus. Energy and Mass Energy has Mass! Equation: E= m= c= wave/particle duality of nature: Ephoton ℎ𝑐 = mphoton = 𝜆 ℎ 𝜆𝑐 Energy changes in the H atom: ΔE = Efinal state – Einital state λ= ℎ𝑐 𝛥𝐸