CH 14-2 Characteristics of Benzene
advertisement

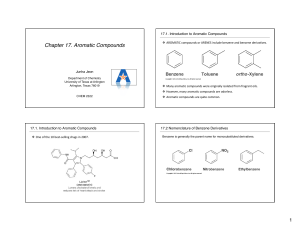
CH 14-2 Characteristics of Benzene •Hydrogen deficiency •All carbons in ring are sp2 hybridized •Geometry •Reactivity of benzene vs alkenes •Benzene is ONE example of a class of compounds characterized as “AROMATIC.” These compounds possess the physical property of “AROMATICITY.” • AROMATICITY is a physical property that explains the unique stability, reactions and toxicity of benzene and related compounds. Aromaticity •Conjugation: each atom of the ring has a p-orbital. •Delocalization: since the p-orbitals are all conjugated, the pi electrons can move freely from one orbital to the next; the pi electrons are delocalized. •Resonance: conjugated, delocalized pi electrons lead to resonance structures: Historical Representations of Benzene •How can we tell if a compound is aromatic? •Huckel’s Rule: Aromaticity exists if # pi electrons = 4n + 2 •Since n = any whole #, there are Huckle #’s of pi electrons for aromatic compounds (n = 0, 1, 2, 3: 2, 6, 10, 14, etc. NH Lone electron pair can count towards aromaticity •Why are these compounds non-aromatic? Aromatic Ions