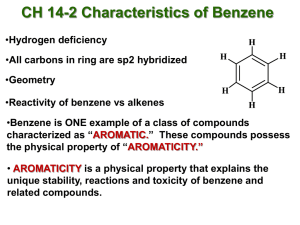
Suez Canal University Faculty of science Student name Karim Nader Ibrahim Mohamed Faculty Science Level Two Department Chemistry National ID 29909060201371 Student code 1801100165 Program Chemistry Subject Organic Chemistry 2 (CH205) 1 Suez Canal University Faculty of science Introduction Discovery of Benzene Benzene was first discovered by British Professor Michael Faraday in 1825, while he was heating whale oil. He found a colorless liquid. The name benzene was given by German Chemist Eilhard Mitscherlich in 1833. The cyclic structure of aromatic compounds remained unknown until 1865 when German Professor Friedrich August Kekulé studied it, Kekulé did not discover the presence of interactions between the double bonds. We had to wait until 1931 for American Professor Linus Pauling to found that benzene has a hybrid structure composed of delocalized electrons. Research objectives Conjugation and resonance. Aromaticity. Aromatic Hydrocarbons. Benefits of aromatic compounds. 2 Suez Canal University Faculty of science Research I. Conjugation and resonance Conjugation is the ability to resonate, so there is no difference between them. this happens when two or more p orbitals overlap each other. Overlap can extend beyond two p orbitals to include three, four, five, and even more consecutive p orbitals. The overlapping can include an empty p orbital or an orbital containing a lone pair or p-orbitals of a pi bond or a half-filled orbital. Resonance of Benzene The carbon (C) atoms in the benzene ring are sp2 hybridized. One C atom which have sp2 hybridized orbitals overlaps with the sp2 orbital of adjacent carbon atom C-C sigma bond. Another sp2 hybridized orbitals of the same Carbon atom combine with s orbital of hydrogen to C-H sigma bond. The unhybridized p orbitals of carbon atoms form Pi bonds with adjacent carbon atoms by lateral overlap. 3 Suez Canal University Faculty of science II. Aromaticity Organic compounds are divided into: Aromatic compounds, Nonaromatic compound and Anti-aromatic compounds. Aromatic compound must be cyclic, planer, completely conjugated, obeys Hückel’s rule. Cyclic: must all atoms be connected with each end. Planer: the overlapping must be Sp2, all atoms in the molecule lie in the same plane. Completely conjugated: The Pi bond must be delocalized in all the ring. Hückel’s rule: Hückel's Molecular Orbital Theory, a compound is stable if all of its orbitals are filled or half filled with paired electrons. The 4n+2 rule, count the number of π electrons in the molecule. Then, make this number equal to 4n+2 and solve for n. If is 0 or any positive integer. For example, benzene has six π electrons: 4 Suez Canal University Faculty of science Each π bond always have 2 π electrons. Benzene has 3 double bonds; therefore, it has 6 π electrons. 5 Suez Canal University Faculty of science III. Aromatic Hydrocarbons Aromatic hydrocarbons are a type of organic compounds. Aromatic compounds contain a straight unsaturated ring of atoms that is stable due to a delocalization of π electrons. The most important aromatic compound is composed of six carbon atoms in the form of a ring. Properties of Aromatic Hydrocarbons The first aromatic hydrocarbon compound was benzene. In the benzene ring each carbon has one H and each two alternative carbon have double bond with alternative with single bond in the other two carbon C—C=C in which the pi electron is delocalized. The continuous delocalization of pi electrons in the benzene molecule is represented by a circle inside the hexagon. These compounds exhibit aromaticity (additional stability granted by resonance), The ratio of carbon atoms to hydrogen atoms is relatively high in these types of molecules, when burnt the aromatic hydrocarbons display a strong and sooty flame which is yellow in color, these compounds generally undergo electrophilic substitutions and nucleophilic aromatic substitution reactions. 6 Suez Canal University Faculty of science IV. Benefits of aromatic compounds Heme: it’s pigment that give the blood it’s red color, which it takes important role in structure of hemoglobin in blood. The molecule heme contains 8 rings connected with each other with 22 π electrons. According to Hückel's 4n + 2 rule, it is aromatic (n = 5). The uses of benzene include making plastics, resins, synthetic fibers, rubber lubricants, dyes, detergents, drugs and pesticides, glues, adhesives, cleaning products, paint strippers and tobacco smoke. Toluene is used in making of paints, alcoholic drinks, glues and nail polish remover. 7 Suez Canal University Faculty of science Dimethylbenzene (xylene) is used in medicine, dentistry and plastics, solvent, clearing agent, lubricant, precursor to polyester. Uses of Aromatic Hydrocarbons 1. 2. 3. 4. 5. Methylbenzene is used as a solvent in some types of glues Naphthalene is used in preservation. For the synthesis of drugs and dyes. TNT is widely used for explosives. Plastic industry (PVC) and petrochemical industries. Conclusion The aromaticity plays an important role in understanding of organic compounds, it makes the compound more stable by resonance of electron in the ring, which make the compound used in several industries. Hückel’s rule make it easy for us to knew that the compound is aromatic or non-aromatic or anti-aromatic. Molecular orbital theory makes it possible to understand the conjugation and the hybridization. The aromatic compounds enter in several industry and in components of our body. 8 Suez Canal University Faculty of science References P. Schleyer, "Aromaticity (Editorial)", Chemical Reviews, 2001. P. Schleyer, "Introduction: Delocalization- and (Editorial)", Chemical Reviews, 2005. Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry: Structure and Function. New York: W.H. Freeman and Company, 2007. https://www.minichemistry.com https://chem.libretexts.org https://www.sciencedirect.com/ 9