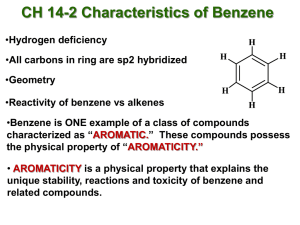
17.1. Introduction to Aromatic Compounds Chapter 17. Aromatic Compounds v AROMATIC compounds or ARENES include benzene and benzene derivatives. Junha Jeon Department of Chemistry University of Texas at Arlington Arlington, Texas 76019 v Many aromatic compounds were originally isolated from fragrant oils. v However, many aromatic compounds are odorless. CHEM 2322 17.1. Introduction to Aromatic Compounds v One of the 10 best-selling drugs in 2007. v Aromatic compounds are quite common. 17.2 Nomenclature of Benzene Derivatives Benzene is generally the parent name for monosubstituted derivatives. 1 17.2 Nomenclature of Benzene Derivatives 17.2 Nomenclature of Benzene Derivatives • Many benzene derivatives have common names. • Many benzene derivatives have common names. • For some compounds, the common name becomes the parent name. • For some compounds, the common name becomes the parent name. 17.2 Nomenclature of Benzene Derivatives If the substituent is larger than the ring, the substituent becomes the parent chain. 17.2 Nomenclature of Benzene Derivatives Aromatic rings are often represented with a Ph (for phenyl) or with a φ (phi) symbol. Aromatic rings are often represented with a Ph (for phenyl) or with a φ (phi) symbol. 2 17.2 Nomenclature of Benzene Derivatives The common name for dimethyl benzene derivatives (disubstituted benzene) is XYLENE. 17.2 Nomenclature of Benzene Derivatives 17.2 Nomenclature of Benzene Derivatives The common name for dimethyl benzene derivatives (disubstituted benzene) is XYLENE. 17.2 Nomenclature of Benzene Derivatives 1. Identify the parent chain (the longest consecutive chain of carbons). 1. Identify the parent chain (the longest consecutive chain of carbons). 2. Identify and name the substituents. 2. Identify and name the substituents. 3. Number the parent chain and assign a locant (and prefix if necessary) to 3. Number the parent chain and assign a locant (and prefix if necessary) to 4. each substituent. each substituent. – Give the first substituent the lowest number possible. – Give the first substituent the lowest number possible. List the numbered substituents before the parent name in alphabetical 4. List the numbered substituents before the parent name in alphabetical order. order. – Ignore prefixes (except iso) when ordering alphabetically. – Ignore prefixes (except iso) when ordering alphabetically. 3 17.2 Nomenclature of Benzene Derivatives 17.2 Nomenclature of Benzene Derivatives 1. Identify the parent chain (the longest consecutive chain of carbons). 1. Identify the parent chain (the longest consecutive chain of carbons). 2. Identify and name the substituents. 2. Identify and name the substituents. 3. Number the parent chain and assign a locant (and prefix if necessary) to 3. Number the parent chain and assign a locant (and prefix if necessary) to 4. each substituent. each substituent. – Give the first substituent the lowest number possible. – Give the first substituent the lowest number possible. List the numbered substituents before the parent name in alphabetical 4. List the numbered substituents before the parent name in alphabetical order. order. – Ignore prefixes (except iso) when ordering alphabetically. – Ignore prefixes (except iso) when ordering alphabetically. 17.2 Nomenclature of Benzene Derivatives 17.2 Nomenclature of Benzene Derivatives 4 17.3 Structure of Benzene 17.3 Structure of Benzene In 1766, August Kekulé proposed that benzene is a ring comprised of alternating In 1766, August Kekulé proposed that benzene is a ring comprised of alternating double and single bonds. double and single bonds. Kekulé suggested that the exchange of double and single bonds was an Kekulé suggested that the exchange of double and single bonds was an equilibrium process. equilibrium (???) process. 17.3 Structure of Benzene 17.4 Stability of Benzene In 1766, August Kekulé proposed that benzene is a ring comprised of alternating • The stability that results from a ring being aromatic is striking. double and single bonds. • Recall that in general, alkenes readily undergo addition reactions. Kekulé suggested that the exchange of double and single bonds was an • Aromatic rings are stable enough that they do not undergo such reactions. equilibrium (???) process. 5 17.4 Stability of Benzene • The stability that results from a ring being aromatic is striking. • Recall that in general, alkenes readily undergo addition reactions. • Aromatic rings are stable enough that they do not undergo such reactions. 17.4 Stability of Benzene Heats of hydrogenation can be used to quantify aromatic stability. 17.4 Stability of Benzene Heats of hydrogenation can be used to quantify aromatic stability. 17.4 Stability of Benzene Stabilization energy: 152 kJ/mol 6 17.4 Stability of Benzene 17.4 Stability of Benzene Source of Stability Source of Stability v Molecular orbital (MO) theory can help us explain aromatic stability. v Molecular orbital (MO) theory can help us explain aromatic stability. v The six atomic p-orbitals of benzene overlap to make six MOs. v The six atomic p-orbitals of benzene overlap to make six MOs. 17.4 Stability of Benzene 17.4 Stability of Benzene The delocalization of the six pi electrons in the three bonding molecular orbitals accounts for the stability of benzene. Hückel’s Rule Does every fully conjugated cyclic compound have aromatic stability? Some fully conjugated cyclic compounds are reactive rather than being stable like benzene. 7 17.4 Stability of Benzene 17.4 Stability of Benzene Hückel’s Rule Does every fully conjugated cyclic compound have aromatic stability? 17.4 Stability of Benzene Hückel’s Rule Does every fully conjugated cyclic compound have aromatic stability? NO! 17.4 Stability of Benzene Hückel’s Rule Does every fully conjugated cyclic compound have aromatic stability? NO! Hückel’s Rule Does every fully conjugated cyclic compound have aromatic stability? NO! 8 17.4 Stability of Benzene 17.4 Stability of Benzene Hückel’s Rule AROMATIC compounds fulfill two criteria: Hückel’s Rule AROMATIC compounds fulfill two criteria: 1. A fully conjugated ring with overlapping p-orbitals 1. A fully conjugated ring with overlapping p-orbitals 2. Meets HÜCKEL’S RULE: an ODD number of electron pairs or 4n+2 total 2. Meets HÜCKEL’S RULE: an ODD number of electron pairs or “4n+2” π electrons where n = 0, 1, 2, 3, 4, etc. 17.4 Stability of Benzene Hückel’s rule using MO theory and Frost circles Let’s consider the MOs for cyclobutadiene. total π electrons where n = 0, 1, 2, 3, 4, etc. 17.4 Stability of Benzene Hückel’s rule using MO theory and Frost circles Let’s consider the MOs for cyclobutadiene. 9 17.4 Stability of Benzene Hückel’s rule using MO theory and Frost circles The instability of the unpaired electrons (similar to free radicals) makes this ANTIAROMATIC. 17.4 Stability of Benzene Hückel’s rule using MO theory and Frost circles A similar MO analysis for cyclooctatetraene (consider four independent double bonds within a cyclooctane ring) suggests that it is also ANTIAROMATIC. 17.4 Stability of Benzene Hückel’s rule using MO theory and Frost circles A similar MO analysis for cyclooctatetraene (consider four independent double bonds within a cyclooctane ring) suggests that it is also ANTIAROMATIC. 17.4 Stability of Benzene Hückel’s rule using MO theory and Frost circles Cyclooctatetraene: v However, if the structure adopts a tub-shaped conformation, it can avoid the antiaromatic instability. v The conjugation does not extend around the entire ring, so the system is neither aromatic nor antiaromatic. 10 17.4 Stability of Benzene 17.4 Stability of Benzene Hückel’s rule using MO theory and Frost circles Predicting the shapes and energies of MOs requires sophisticated mathematics, but we can use FROST CIRCLES to predict the relative MO energies. Hückel’s rule using MO theory and Frost circles v Use the FROST CIRCLES below to explain the 4n+2 rule. v Note that the number of bonding orbitals is always an odd number; aromatic compounds will always have an odd number of electron pairs. 17.4 Stability of Benzene 17.5 Aromatic Compounds Other Than Benzene Hückel’s rule using MO theory and Frost circles v Use the FROST CIRCLES below to explain the 4n+2 rule. v Note that the number of bonding orbitals is always an odd number; aromatic compounds will always have an odd number of electron pairs. The Criteria for Aromaticity AROMATIC compounds fulfill two criteria: 1. A fully conjugated ring with overlapping p-orbitals 2. Meets HÜCKEL’S RULE: an ODD number of electron pairs or 4n+2 total π electrons where n = 0, 1, 2, 3, 4, etc. ANTIAROMATIC compounds fulfill two criteria 1. A fully conjugated ring with overlapping p-orbitals 2. An EVEN number of electron pairs or 4n total π electrons where n = 0, 1, 2, 3, 4, etc. 11 17.5 Aromatic Compounds Other Than Benzene The Criteria for Aromaticity Compounds that do not contain a ring comprised of continuously overlapping p orbitals. à “nonaromatic” 17.5 Aromatic Compounds Other Than Benzene The Criteria for Aromaticity v Annulenes are rings that are fully conjugated. v Some annulenes are aromatic, while others are antiaromatic. v [10]Annulene is neither. WHY? 17.5 Aromatic Compounds Other Than Benzene The Criteria for Aromaticity 17.5 Aromatic Compounds Other Than Benzene The Criteria for Aromaticity v Annulenes are rings that are fully conjugated. v Annulenes are rings that are fully conjugated. v Some annulenes are aromatic, while others are antiaromatic. v Some annulenes are aromatic, while others are antiaromatic. v [10]Annulene is neither. WHY? v How about [10]Annulene and [14]annulene? 12 17.5 Aromatic Compounds Other Than Benzene 17.5 Aromatic Compounds Other Than Benzene Aromatic Ions v Some rings must carry a formal charge to be aromatic. Aromatic Ions v Some rings must carry a formal charge to be aromatic. v Consider a 5-membered ring. If six pi electrons are present, a 5-membered ring will be aromatic! 17.5 Aromatic Compounds Other Than Benzene 17.5 Aromatic Compounds Other Than Benzene Aromatic Ions Aromatic Ions v Some rings must carry a formal charge to be aromatic. v Some rings must carry a formal charge to be aromatic. v Consider a 5-membered ring. If six pi electrons are present, a 5-membered v Consider a 5-membered ring. If six pi electrons are present, a 5-membered ring will be aromatic! ring will be aromatic! cyclopentadienyl anion: an especially stable anion 1. 2. cyclopentadienyl anion: an especially stable anion resonance stabilized aromatic (continuously overlapping p orbitals and 4n+2 total π electrons) 1. 2. resonance stabilized aromatic (continuously overlapping p orbitals and 4n+2 total π electrons) 13 17.5 Aromatic Compounds Other Than Benzene Via pKa Values to Compare Acidity: pKa Aromatic Ions cyclopentadienyl anion: an especially stable anion 1. 2. resonance stabilized aromatic (continuously overlapping p orbitals and 4n+2 total π electrons): the remarkable acidity of cyclopentadiene – next slides Via pKa Values to Compare Acidity: pKa Via pKa Values to Compare Acidity: pKa 14 17.5 Aromatic Compounds Other Than Benzene 17.5 Aromatic Compounds Other Than Benzene Aromatic Ions cyclopentadienyl anion: an especially stable anion 1. 2. resonance stabilized aromatic (continuously overlapping p orbitals and 4n+2 total π electrons): Aromatic Ions v Consider a 7-membered ring. v If six pi electrons are present, what charge will be necessary to achieve aromaticity? hint: we need 6 electrons. the remarkable acidity of cyclopentadiene (draw molecular geometry) 17.5 Aromatic Compounds Other Than Benzene 17.5 Aromatic Compounds Other Than Benzene Aromatic Ions Aromatic Ions v This cation (tropylium cation) is resonance stabilized. v This cation (tropylium cation) is resonance stabilized. v Aromatic (continuously overlapping p orbitals and 4n+2 total π electrons): a v Aromatic (continuously overlapping p orbitals and 4n+2 total π electrons): a remarkably stable cation remarkably stable cation 15 17.5 Aromatic Compounds Other Than Benzene 17.5 Aromatic Compounds Other Than Benzene Aromatic Heterocycles v Heteroatoms (atoms other than C or H) can also be part of an aromatic ring. Aromatic Heterocycles v Heteroatoms (atoms other than C or H) can also be part of an aromatic ring: e.g. pyridine 17.5 Aromatic Compounds Other Than Benzene Reminder: 17.5 Aromatic Compounds Other Than Benzene The Criteria for Aromaticity Aromatic Heterocycles v Heteroatoms (atoms other than C or H) can also be part of an aromatic ring: e.g. pyridine AROMATIC compounds fulfill two criteria: 1. A fully conjugated ring with overlapping p-orbitals 2. Meets HÜCKEL’S RULE: an ODD number of electron pairs or 4n+2 total π electrons where n = 0, 1, 2, 3, 4, etc. ANTIAROMATIC compounds fulfill two criteria 1. A fully conjugated ring with overlapping p-orbitals 2. An EVEN number of electron pairs or 4n total π electrons where n = 0, 1, 2, 3, 4, etc. 16 17.5 Aromatic Compounds Other Than Benzene 17.5 Aromatic Compounds Other Than Benzene Aromatic Heterocycles Aromatic Heterocycles v If the heteroatom’s lone pair is necessary, it will be included in the HÜCKEL v If the heteroatom’s lone pair is necessary, it will be included in the HÜCKEL number of pi electrons: pyrrole 17.5 Aromatic Compounds Other Than Benzene Polycyclic Aromatic Hydrocarbons (PAHs) The molecules below meet the criteria for aromaticity: 1. A fully conjugated ring with overlapping p-orbitals 2. Meets HÜCKEL’S RULE: an ODD number of electron pairs or 4n+2 total π number of pi electrons: pyrrole 17.5 Aromatic Compounds Other Than Benzene Polycyclic Aromatic Hydrocarbons (PAHs) v Many polycyclic compounds are also aromatic. electrons where n = 0, 1, 2, 3, 4, etc. 17 17.6 Reactions at the Benzylic Position 17.6 Reactions at the Benzylic Position • A carbon that is attached to a benzene ring is BENZYLIC. • A carbon that is attached to a benzene ring is BENZYLIC. • Recall that aromatic rings and alkyl groups are not easily oxidized. • Recall that aromatic rings and alkyl groups are not easily oxidized. 17.6 Reactions at the Benzylic Position 17.6 Reactions at the Benzylic Position • A carbon that is attached to a benzene ring is BENZYLIC. • A carbon that is attached to a benzene ring is BENZYLIC. • Recall that aromatic rings and alkyl groups are not easily oxidized. • Recall that aromatic rings and alkyl groups are not easily oxidized. • In general, benzylic positions can readily be fully oxidized. • In general, benzylic positions can readily be fully oxidized. For example, 18 17.6 Reactions at the Benzylic Position 17.6 Reactions at the Benzylic Position • A carbon that is attached to a benzene ring is BENZYLIC. • A carbon that is attached to a benzene ring is BENZYLIC. • Recall that aromatic rings and alkyl groups are not easily oxidized. • Recall that aromatic rings and alkyl groups are not easily oxidized. • In general, benzylic positions can readily be fully oxidized. • In general, benzylic positions can readily be fully oxidized. • Permanganate can also be used as an oxidizing reagent. 17.6 Reactions at the Benzylic Position • A carbon that is attached to a benzene ring is BENZYLIC. 17.6 Reactions at the Benzylic Position • Substitution reactions of benzylic halides (SN1 and SN2) • Recall that aromatic rings and alkyl groups are not easily oxidized. • In general, benzylic positions can readily be fully oxidized. • Permanganate can also be used as an oxidizing reagent. • BENZYLIC positions have similar reactivity to allylic positions: Benzylic positions readily undergo free radical bromination. 19 17.6 Reactions at the Benzylic Position • Substitution reactions of benzylic halides (SN1 and SN2 and E1) 17.6 Reactions at the Benzylic Position • Substitution reactions of benzylic halides (E1 and E2) 17.6 Reactions at the Benzylic Position • Substitution reactions of benzylic halides (SN1 and SN2) and E1) 17.6 Reactions at the Benzylic Position • Substitution reactions of benzylic halides (E1 and E2) 20 17.7 Reduction of the Aromatic Moiety • Under forceful conditions, benzene can be reduced to cyclohexane. 17.7 Reduction of the Aromatic Moiety • Under forceful conditions, benzene can be reduced to cyclohexane. • Vinyl side groups can be selectively reduced. • ΔH is just slightly less than the expected –120 kJ/mol expected for a C=C à C–C conversion. • 17.7 Reduction of the Aromatic Moiety • Can you recall dissolving metal reduction? WHY are less forceful conditions required? Dissolving metal reduction Sodium Metal Serves as a Source of Electrons 21 Dissolving Metal Reduction Dissolving Metal Reduction Dissolving Metal Reduction 17.7 Reduction of the Aromatic Moiety • Can you recall dissolving metal reduction? • Birch reduction is mechanistically similar to dissolving metal reduction (utilizing almost identical reaction condition). Do the pKa values for NH3 and the alkene favor the proton transfer? 22 17.7 Reduction of the Aromatic Moiety 17.7 Reduction of the Aromatic Moiety • Can you recall dissolving metal reduction? • Can you recall dissolving metal reduction? • Birch reduction is mechanistically similar to dissolving metal reduction • Birch reduction is mechanistically similar to dissolving metal reduction (utilizing almost identical reactioncondition). 17.7 Reduction of the Aromatic Moiety • (utilizing almost identical reactioncondition). 17.7 Reduction of the Aromatic Moiety Note that the BIRCH reduction product has sp3 hybridized carbons on The presence of an electron donating alkyl side group provides regioselectivity. opposite ends of the ring. HOW? 23 17.7 Reduction of the Aromatic Moiety 17.8 Spectroscopy of Aromatic Compounds The presence of an electron withdrawing carbonyl side group provides different regioselectivity. HOW? 17.8 Spectroscopy of Aromatic Compounds 17.8 Spectroscopy of Aromatic Compounds v 1H NMR 24 17.8 Spectroscopy of Aromatic Compounds v 1H NMR 17.8 Spectroscopy of Aromatic Compounds v 13C NMR 25