An Aldol Condensation Reaction: The Synthesis of Tetraphenylcyclopentadienone—An
advertisement

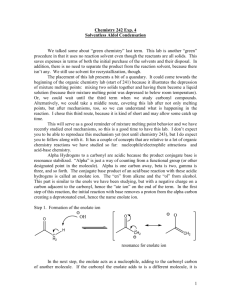
An Aldol Condensation Reaction: The Synthesis of Tetraphenylcyclopentadienone—An Example of a Double-Crossed Aldol Addition Reactions of Carbonyl Groups The chemical reactivity of aldehydes and ketones is closely associated with the presence of the carbonyl group in their structures. For example, both aldehydes and ketones undergo addition reactions such as the addition of a Grignard reagent to the carbonyl group as shown in Figure 1. O RCH + R'MgX aldehyde O RCR'' + R'MgX ketone addition Grignard reagent OMgX RCH R' salt addition Grignard reagent OMgX RCR'' R' salt H+ H2O acidification H+ H2O acidification OH RCH R' IIo alcohol OH RCR'' R' IIIo alcohol Figure 1. Addition reactions. The reactions in Figure 1 differ only because the pink H of an aldehyde is replaced by R′′ in the ketone. The addition reaction occurs at the carbonyl group. The carbonyl group is polarized so that the carbon atom bears a partial positive charge and the oxygen atom bears a partial negative charge. The R′ group of the Grignard reagent is negative relative to the positive Mg atom. Thus, the negative R′ group bonds to the positive carbon atom, and the negative oxygen and metallic magnesium form an ionic bond, yielding a salt in each reaction. The addition product is acidified in each case to make a covalent alcohol. The aldehyde produces a IIo alcohol; whereas, the ketone produces a IIIo alcohol owing to the R′′ group. The two equations for addition reactions in Figure 1 are summarized in Figure 2. A nucleophile (negative species) bonds to the carbonyl carbon (positive), breaking the bond of the carbonyl group. Lab 12 1 O- O :Nu C C Nu Figure 2. Addition of a nucleophile to a carbonyl group. Figure 2 focuses our attention on the salient part of an addition reaction that involves either a ketone or an aldehyde. A nucleophile bonds to the carbonyl carbon. In an aldol addition reaction, the nucleophile is an enolate formed from an aldehyde or ketone by the removal of a hydrogen atom next to the carbonyl group. The enolate (negative nucleophile) then adds to a carbonyl group of another aldehyde or ketone as shown in Figure 1. Formation of an Enolate from an Aldehyde or Ketone Aldehydes and ketones that possess alpha hydrogen atoms can form enolates. The Greek alphabet (, , etc.) is used by chemists to identify carbon atoms in relation to the carbon atom of a carbonyl group. An alpha carbon atom is a carbon atom that is bonded directly to the carbon atom of a carbonyl group. A beta carbon atom is the second carbon atom from the carbonyl carbon, a gamma carbon is the third carbon away from the carbonyl, etc. Likewise, hydrogen atoms are named according to the name of the carbon atom to which they are bonded. A hydrogen atom bonded to an alpha carbon is called an alpha hydrogen, etc. Figure 3 shows these relationships for acetaldehyde and 2pentanone. carbon 3 hydrogens H O H C C H H acetaldehyde Lab 12 2 hydrogens 3 hydrogens -C -C H H H O H H C C C C C H H H H H 2-pentanone 2 Figure 3. Alpha hydrogens. Alpha hydrogen atoms are important because they can be removed by a base. Thus, the hydrogen atoms shown in blue and pink in Figure 3 can be removed in base. What is the first thing that happens when acetaldehyde is placed in base? The base removes or abstracts a hydrogen atom. The base forms a new bond with a pair of electrons from the base, so the hydrogen atom leaves its electrons with the alpha carbon atom. Thus, the base abstracts H+ or a proton, leaving C- or a carbanion. Compounds that contain only one kind of equivalent alpha hydrogens (e.g., acetaldehyde has 3 equivalent blue H atoms) are preferred over compounds that contain two kinds of equivalent alpha hydrogens (e.g., 2pentanone has 2 pink and 3 blue equivalent H atoms). Figure 4 shows the first step when acetaldehyde reacts with hydroxide ion, which may come from sodium hydroxide or potassium hydroxide. H O H C C H H -OH step 1 acetaldehyde O H C C H + HOH H carbanion Figure 4. Formation of carbanion. A base abstracts an alpha hydrogen atom to form a carbanion. The carbanion is a nucleophile, which can react with a carbonyl group as shown in Figure 2. The carbanion shown in Figure 4 is also an enolate because of resonance. Figure 5 shows how the initially formed carbanion from acetaldehyde is resonance stabilized via the enolate. Thus, alpha hydrogen atoms are acidic because the resulting anion is resonance stabilized. Lab 12 3 O H C C H OH C C H H H carbanion enolate Figure 5. Resonance structures of carbanion and enolate. Self-Aldol Addition Reaction The carbanion or enolate of Figure 5 is a nucleophile, which can add to a carbonyl group of an aldehyde or ketone. When an enolate made from an aldehyde such as acetaldehyde reacts with another molecule of acetaldehyde, the addition product is a dimer and the reaction is called a self-aldol addition reaction. Figure 6 shows the carbanion (enolate) of Figure 5 adding to a second molecule of acetaldehyde. H O H C C H + H acetaldehyde H OH H OO H-OH H C C H + -OH H C C H H C C H step 2 H CH2CH H H CH2CH step 3 O O carbanion aldol of acetaldehyde Figure 6. Synthesis of aldol. Steps 1-3 of figures 4 and 6 show the complete mechanism of an aldol addition reaction. In Step 1, base abstracts an alpha hydrogen to make an enolate. In Step 2, the enolate adds to the carbonyl of a second molecule, forming an oxyanion, which abstracts a proton from water to make 3-hydroxybutanal. The hydrogen atom that is abstracted in Step 3 replaces the H atom that was abstracted in Step 1, so the product is a dimer of the starting compound. 3-Hydroxybutanal is the first member of a family of compounds called aldols. Aldols are so named because they contain an aldehyde and an alcohol, and the alcohol hydroxyl group is bonded to a carbon atom that is two carbon atoms away from the carbonyl Lab 12 4 carbon atom. The structural feature in an aldol is a-hydroxycarbonyl. Any aldehyde or ketone with a -hydroxyl group is an aldol. Figure 7 shows examples of aldols. O O OH OH O H OH Figure 7. Aldols. Acetaldehyde is the simplest compound that has alpha hydrogens. Therefore, the common name of the aldol made the self-aldol addition reaction of acetaldehyde is called aldol. All other aldehydes or ketones that contain the hydroxycarbonyl structure are members of the aldol family and are called aldols. Crossed-Aldol Addition Reaction In a self-aldol addition reaction, both reactants come from the same compound. When the two reactants in Step 2 come from different compounds, the reaction is called a crossed-aldol addition reaction. A crossed-aldol addition reaction generally involves one aldehyde or ketone without alpha hydrogens and one with alpha hydrogens; therefore, only the compound with alpha hydrogens can form an enolate in base. If both compounds contained alpha hydrogens, then multiple products would be produced. The enolate is the nucleophile that adds to the carbonyl group of the compound without alpha hydrogens. Benzaldehyde contains no alpha hydrogens and acetone contains six equivalent alpha hydrogens; therefore, these two compounds can undergo a crossed-aldol addition reaction without forming multiple products. Figure 8 shows the structures of benzaldehyde and acetone. You should know the structures of these compounds. O C not H benzaldehyde Lab 12 O CH3CCH3 acetone 5 Figure 8. Benzaldehyde and acetone. By inspecting the structures in Figure 8, we see that benzaldehyde has no alpha hydrogen atoms and acetone does. Thus, when these compounds are placed in base, acetone will form an enolate by the mechanism shown in Figure 4. The enolate serves as the nucleophile that adds to the carbonyl group of benzaldehyde, which serves as the substrate in the addition reaction. Figure 9 shows the reaction. O CH -OH O CH3CCH3 Step 1 O -CH CCH 2 3 Step 2 OCH HOH CH2CCH3 Step 3 O OH CH CH2CCH3 O Figure 9. Synthesis of an aldol. In Step 1, base removes an alpha hydrogen atom from acetone (the only reactant with alpha hydrogens), forming the cabanion or enolate that serves as the nucleophile. In Step 2, the negative carbanion adds to the positive carbonyl carbon to form the oxyanion. In Step 3, the adduct from Step 2 abstracts a proton from solvent to form the aldol (-hydroxyketone) product. Note that the aldol addition and Grignard reactions are similar; they differ only in how the nucleophile is made. The nucleophile in an aldol reaction is formed from an aldehyde or ketone with alpha hydrogens; the nucleophile of a Grignard reagent is formed from an alkyl halide. Aldol Condensation Reaction We learned first semester that alcohols can eliminate water to form an alkene. Aldols are alcohols, and they can also eliminate water to form alkenes. Thus, initially formed aldols eliminate water when they are heated, generally in acid. Recall that a mineral acid protonates a hydroxyl oxygen atom, making a good leaving group. Consider aldol itself. Figure 10 shows that two different alkenes are possible when water is eliminated. In one alkene, the hydroxyl group and another alpha hydrogen are eliminated. In the second alkene, the hydroxyl group and a gamma hydrogen are eliminated. Lab 12 6 H H C C H OH H O H C C C C H H H H aldol Step 4 H H X H H C H O C C H -unsaturated aldehyde (conjugated, more stable) H O C C H H C H -unsaturated aldehyde (unconjugated, less stable) Figure 10. Dehydration of aldol. The dehydration of aldol gives only the -unsaturated aldehyde, because it is more stable than the -unsaturated aldehyde. When an aldol is heated, it dehydrates to give an -unsaturated aldehyde or ketone. Thus, an aldol reaction may be stopped after Step 3 to give an aldol, or after Step 4 to give an unsaturated aldehyde or ketone. When the reaction sequence includes Step 4, the overall reaction is an aldol-condensation reaction. Condensation implies the loss of water (or another small molecule such as ethanol) from two organic reactants. Figure 11 shows Step 4 that changes the aldol addition in Figure 9 into an aldolcondensation reaction. OH CH CH2CCH3 O - H2O O CH CHCCH3 aldol -unsaturated ketone Figure 11. Conversion of an aldol to an -unsaturated ketone. The Experimental Reaction Lab 12 7 In this experiment, we conduct an interesting aldol condensation that involves one ketone that has -hydrogen atoms (dibenzyl ketone) and one that does not (benzil). Thus, dibenzyl ketone will serve as the nucleophile, because it will donate a proton to a base to form an enolate. The overall reaction is shown in Figure 12. O O O C C benzil + O CH2 C CH2 dibenzyl ketone KOH - H2O tetraphenylcyclopenatadienone Figure 12. Double-crossed aldol condensation. This reaction involves two different ketones, so the reaction is described as a crossed-aldol condensation. Note that we have formed two -unsaturated groupings, so the crossed aldol reaction occurs twice. Thus, the reaction can be called a double-crossed aldol condensation. You can work out the mechanism for the reaction by forming an enolate from dibenzyl ketone in base, allowing it to attack on carbonyl group in benzil, and eliminating water from the adduct. Repeat the process with the remaining -hydrogen atoms while closing the five-membered ring. The final product is a purple solid. Procedure 1. Set up a sand bath on your lab bench. [Metallic heating mantle, sand, and rheostat] Adjust the setting on the rheostat to 70. 2. Add 0.1-g benzil, 0.1-g dibenzyl ketone, 1.5-mL absolute ethanol, and a tiny boiling chip to a 15-mL round-bottomed flask. [Use a creased, glassine paper to transfer the solids.] 3. Attach a condenser from a micro kit to the round-bottomed flask with the blue adapter from the kit. 4. Affix the condenser-flask to a ring stand so that the round-bottom flask is slightly into the sand of the sand bath. Lab 12 8 5. Heat the mixture on the sand bath until the solids dissolve but do not let the ethanol boil (bp = 78 oC). 6. Slowly add seven drops of 5% (weight-volume) alcoholic potassium hydroxide (KOH) through the condenser. 7. Bring the ethanol to a boil and allow the mixture to reflux for 10 min. 8. If a solid begins to form and cake on the round-bottomed flask, add a few drops of absolute ethanol to keep the solid moist. 9. While the mixture is under reflux, prepare an ice-water bath in a beaker large enough to hold the round-bottomed flask. 10. Remove the apparatus from the sand bath and allow the round-bottomed flask to cool to room temperature. 11. Place the flask, still attached to the condenser, in the ice-water bath and allow the flask to come to the temperature of the ice bath. 12. Remove the condenser and collect the crystals on a Büchner funnel. 13. Continue to apply suction until the crystals are as dry as possible. 14. Weigh the crystals and record the yield directly in your notebook. 15. Show the crystals to your instructor, who will tell you what to do with them. 16. Clean your work area. Replace all equipment to the correct storage location. Lab 12 9 Lab 12 10 Aldol Condensation Questions Last name___________________________, First name________________________ 1. Circle the structures below that represent compounds that can undergo self-aldol condensations. (Get all correct for one point.) O CH2O CH3CHO CH3CCH3 CH2O2 O C CHO 2. Write an equation for the enolization of acetaldehyde in an acidic medium. 3. Write an equation for the enolate formation of acetone in base (OH-). 4. Write an equation for the self-aldol addition (not condensation) of acetaldehyde. 5. Circle the following stuctures that have -hydrogen atoms. O CCH2 O HCH CHO CH2CHO Complete the following equations. O CH 6. 7. O C (1)OH+ CH3CHO (2) heat (1)OH+ CH3CHO (2) heat 8. O C + O CH3CCH3 (1)OH(2) heat (1)OH9. O (2) heat 10. Why was your product in lab a colored compound? Lab 12 11